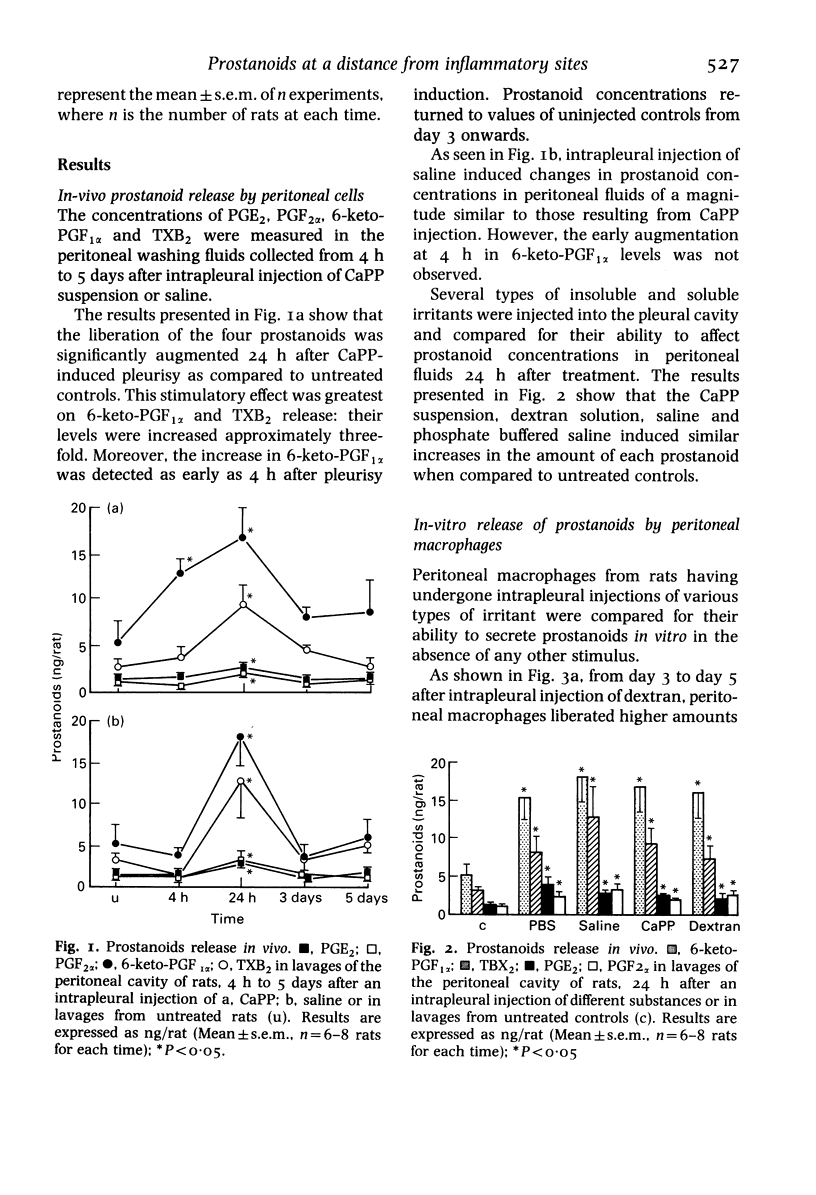
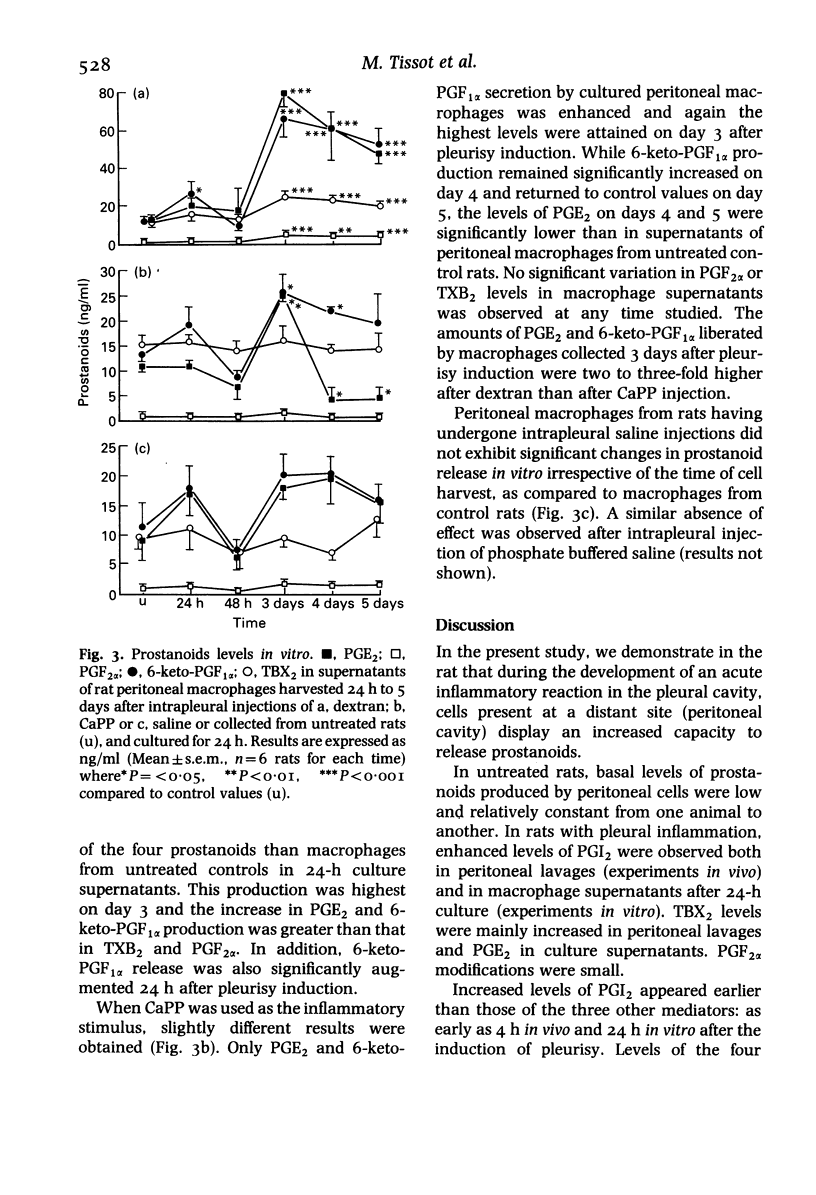
Abstract
During the development of an acute inflammatory reaction induced in the rat pleural cavity by dextran, calcium pyrophosphate, saline or phosphate buffered saline, macrophages present at a distant site (peritoneal cavity) display an increased capacity to release prostanoids: prostaglandins, prostacyclin and thromboxane. Enhanced levels of 6-keto-PGF1 alpha were observed both in peritoneal lavages (experiments in vivo) and in macrophage supernatants after 24-h culture (experiments in vitro). TXB2 levels were mainly increased in peritoneal lavages and PGE2 in culture supernatants. In vivo, levels of prostanoids in the peritoneal cavity reached a maximum 24 h after the induction of pleurisy whatever the injected substance. In vitro, amounts of arachidonic acid metabolites were highest in supernatants of cultured peritoneal macrophages harvested 72 h after the pleural injection of dextran or CaPP. These results show that the regulation of macrophage functions is closely related to prostanoid production, especially the release of PGE2 and PGI2.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazan H. E., Birkle D. L., Beuerman R. W., Bazan N. G. Inflammation-induced stimulation of the synthesis of prostaglandins and lipoxygenase-reaction products in rabbit cornea. Curr Eye Res. 1985 Mar;4(3):175–179. doi: 10.3109/02713688509000847. [DOI] [PubMed] [Google Scholar]
- Bird J., Giroud J. P. The reactivity of neutrophils at the site of an acute inflammatory reaction as measured by chemiluminescence. Agents Actions. 1984 Oct;15(3-4):349–355. doi: 10.1007/BF01972370. [DOI] [PubMed] [Google Scholar]
- Bird J., Lay J. C., Lee H. J. The effects of new local anti-inflammatory steroids on leucocyte migration and prostanoid liberation in rats. J Pharm Pharmacol. 1986 Aug;38(8):589–594. doi: 10.1111/j.2042-7158.1986.tb03086.x. [DOI] [PubMed] [Google Scholar]
- Bird J., Sheng Y. J., Florentin I., Giroud J. P. Release of interleukin I and low-molecular-weight lymphocyte-activating factors by rat peritoneal macrophages and its enhancement by acute non-specific inflammatory processes. Br J Exp Pathol. 1985 Jun;66(3):271–277. [PMC free article] [PubMed] [Google Scholar]
- Bird J., Tissot M., Giroud J. P. The modulation of peritoneal macrophage chemiluminescence by acute pleural inflammation, prostanoids and cyclo/lipoxygenase inhibitors. Agents Actions. 1985 Dec;17(2):184–191. doi: 10.1007/BF01966590. [DOI] [PubMed] [Google Scholar]
- Bockman R. S. Prostaglandin production by human blood monocytes and mouse peritoneal macrophages: synthesis dependent on in vitro culture conditions. Prostaglandins. 1981 Jan;21(1):9–31. doi: 10.1016/0090-6980(81)90192-1. [DOI] [PubMed] [Google Scholar]
- Brunda M. J., Herberman R. B., Holden H. T. Inhibition of murine natural killer cell activity by prostaglandins. J Immunol. 1980 Jun;124(6):2682–2687. [PubMed] [Google Scholar]
- Cook J. A., Wise W. C., Halushka P. V. Thromboxane A2 and prostacyclin production by lipopolysaccharide-stimulated peritoneal macrophages. J Reticuloendothel Soc. 1981 Nov;30(5):445–450. [PubMed] [Google Scholar]
- Di Rosa M., Giroud J. P., Willoughby D. A. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971 May;104(1):15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Roubin R., Petit J. F., Benveniste J. Lipid-mediator synthesis in peritoneal macrophages from mice injected with immunostimulants. Biochim Biophys Acta. 1983 Mar 22;751(1):90–98. doi: 10.1016/0005-2760(83)90260-6. [DOI] [PubMed] [Google Scholar]
- Florentin I., Bird J., Le Garrec Y., Chung V., Giroud J. P. Modifications of host defence mechanisms by an acute non-immunological inflammatory reaction. Br J Exp Pathol. 1985 Jun;66(3):257–270. [PMC free article] [PubMed] [Google Scholar]
- Gemsa D., Leser H. G., Seitz M., Deimann W., Bärlin E. Membrane perturbation and stimulation of arachidonic acid metabolism. Mol Immunol. 1982 Oct;19(10):1287–1296. doi: 10.1016/0161-5890(82)90295-4. [DOI] [PubMed] [Google Scholar]
- Glatt M., Kälin H., Wagner K., Brune K. Prostaglandin release from macrophages: an assay system for anti-inflammatory drugs in vitro. Agents Actions. 1977 Sep;7(3):321–326. doi: 10.1007/BF01969563. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Henderson B., Higgs G. A., Moncada S., Salmon J. A. Synthesis of eicosanoids by tissues of the synovial joint during the development of chronic erosive synovitis. Agents Actions. 1986 Jan;17(3-4):360–362. doi: 10.1007/BF01982646. [DOI] [PubMed] [Google Scholar]
- Humes J. L., Bonney R. J., Pelus L., Dahlgren M. E., Sadowski S. J., Kuehl F. A., Jr, Davies P. Macrophages synthesis and release prostaglandins in response to inflammatory stimuli. Nature. 1977 Sep 8;269(5624):149–151. doi: 10.1038/269149a0. [DOI] [PubMed] [Google Scholar]
- Hänsch G. M., Seitz M., Martinotti G., Betz M., Rauterberg E. W., Gemsa D. Macrophages release arachidonic acid, prostaglandin E2, and thromboxane in response to late complement components. J Immunol. 1984 Oct;133(4):2145–2150. [PubMed] [Google Scholar]
- Ohuchi K., Yoshino S., Kanaoka K., Tsurufuji S., Levine L. A possible role of arachidonate metabolism in allergic air pouch inflammation in rats. Anti-inflammatory effect of indomethacin and dexamethasone and the level of prostaglandin E2 in the exudate. Int Arch Allergy Appl Immunol. 1982;68(4):326–331. doi: 10.1159/000233121. [DOI] [PubMed] [Google Scholar]
- Pelletier M., Willoughby D. A., Giroud J. P. Modulating effect of levamisole on DNA synthesis in macrophages in vitro. J Reticuloendothel Soc. 1978 Oct;24(4):333–338. [PubMed] [Google Scholar]
- Sedgwick A. D., Lees P. Studies of eicosanoid production in the air pouch model of synovial inflammation. Agents Actions. 1986 Jun;18(3-4):429–438. doi: 10.1007/BF01965008. [DOI] [PubMed] [Google Scholar]
- Sultan A. M., Dunn C. J., Mimms P. C., Giroud J. P., Willoughby D. A. The leucocoyte disappearance reaction in non-immune acute inflammation. J Pathol. 1978 Dec;126(4):221–230. doi: 10.1002/path.1711260406. [DOI] [PubMed] [Google Scholar]
- Tissot M., Bonne C., Martin B., Solier M., Giroud J. P. Prostacyclin and thromboxanes in carrageenan-induced pleurisy in the rat. Agents Actions. 1984 Jan;14(1):76–81. doi: 10.1007/BF01966837. [DOI] [PubMed] [Google Scholar]
- Tissot M., D'Asniere M., Solier M., Giroud J. P., Engler R. Study of the evolution of acute phase reactants and of thromboxane and prostacyclin during calcium pyrophosphate-induced pleurisy in the rat. Agents Actions. 1984 Jan;14(1):82–87. doi: 10.1007/BF01966838. [DOI] [PubMed] [Google Scholar]
- Velo G. P., Dunn C. J., Giroud J. P., Timsit J., Willoughby D. A. Distribution of prostaglandins in inflammatory exudate. J Pathol. 1973 Nov;111(3):149–158. doi: 10.1002/path.1711110302. [DOI] [PubMed] [Google Scholar]
- Willoughby D. A., Dunn C. J., Yamamoto S., Capasso F., Deporter D. A., Giroud J. P. Calcium pyrophosphate-induced pleurisy in rats: a new model of acute inflammation. Agents Actions. 1975 Feb;5(1):35–38. doi: 10.1007/BF02027156. [DOI] [PubMed] [Google Scholar]


