Abstract
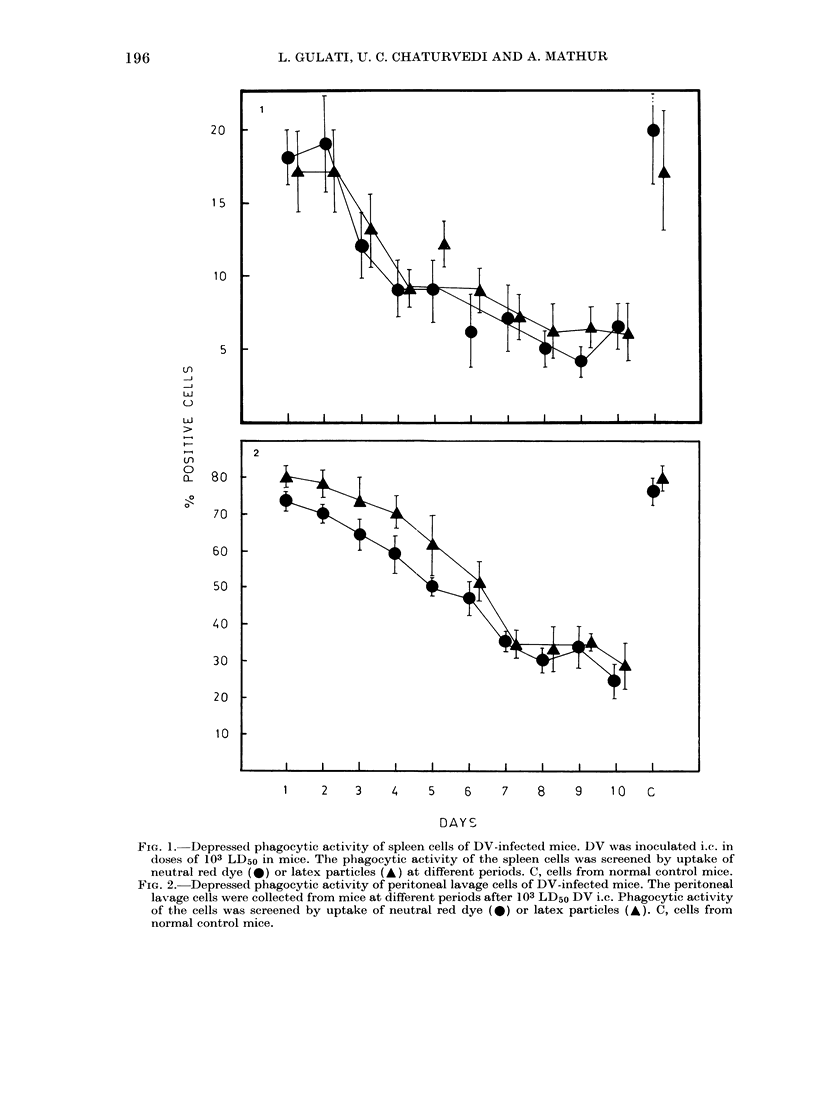
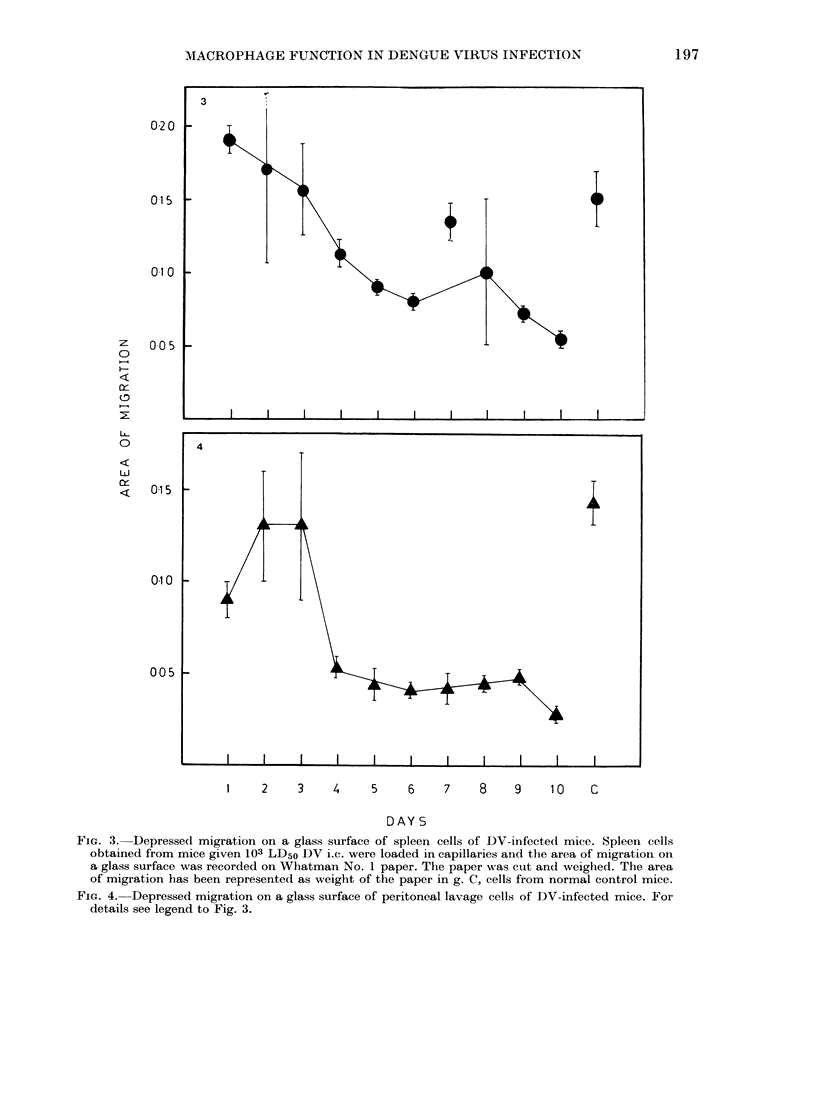
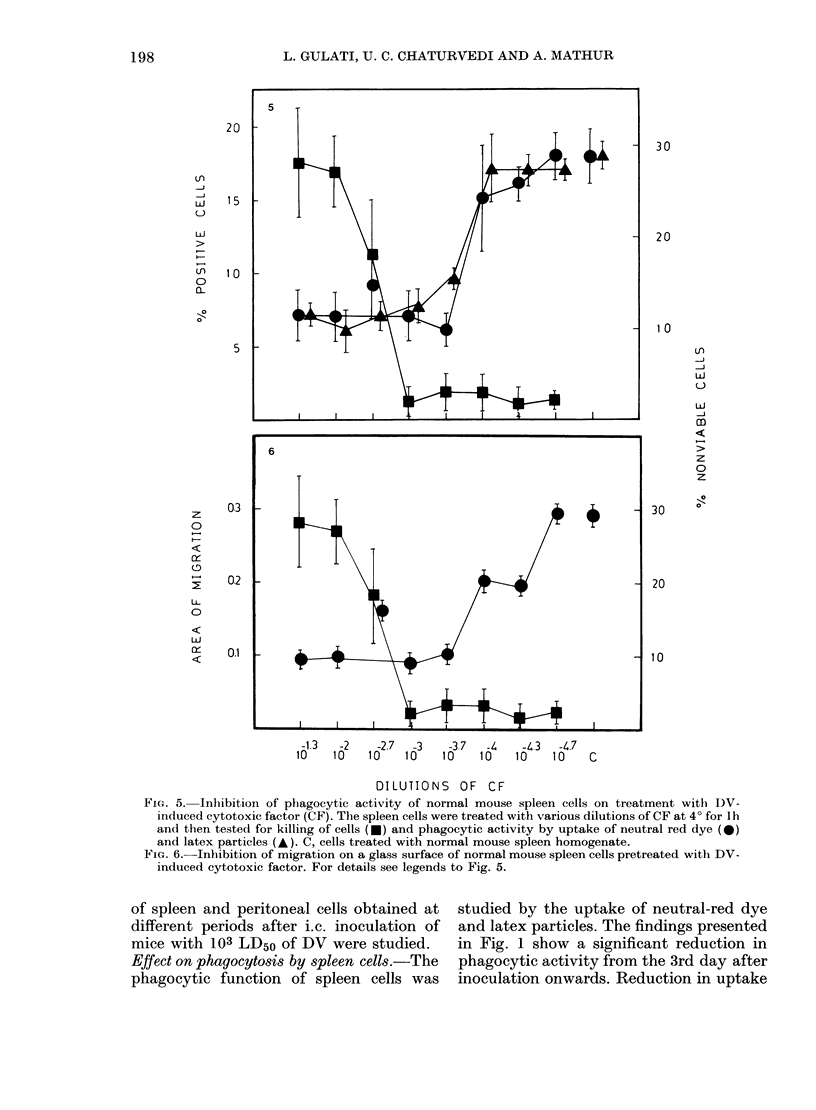
Dengue virus Type 2 (DV) infection causes immunosuppression in mice. Since macrophages are crucial for immune response, we have studied their functions in this condition and report our findings here. It was observed that in DV-infected mice the phagocytosis of neutral-red and latex particles by splenic and peritoneal-cavity macrophages was significantly reduced (P less than 0.001) from Days 3 to 10 after inoculation. Similarly the migration of splenic and peritoneal macrophages on a glass surface was reduced significantly (P less than 0.001) from Days 4 to 10 after inoculation. Pre-treatment of normal mouse spleen cells with DV-induced cytotoxic factor (CF) inhibited the phagocytic and migratory functions in the same way as observed in DV-infected mice. Higher dilutions of CF (10(-3) and 10(-3.7)) did not kill the cells but affected their functions. It was concluded that macrophage functions are affected by killing and metabolic changes in these cells by DV-induced CF, thus producing immunosuppression.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal D. K., Tandon P., Chaturvedi U. C., Kumar A. Biochemical study of certain enzymes and metabolites of the carbohydrate metabolism in the skeletal muscle of the dengue virus-infected mice. J Gen Virol. 1978 Aug;40(2):399–408. doi: 10.1099/0022-1317-40-2-399. [DOI] [PubMed] [Google Scholar]
- Albrecht R. M., Hinsdill R. D., Sandok P. L., Horowitz S. D. Murine macrophage-lymphocyte interactions: scanning electron microscopic study. Infect Immun. 1978 Jul;21(1):254–268. doi: 10.1128/iai.21.1.254-268.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. I. COMPARATIVE ENZYMOLOGY, ISOLATION, AND PROPERTIES. J Exp Med. 1963 Dec 1;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Dalakoti H., Mathur A. Characterization of the cytotoxic factor produced in the spleen of dengue virus-infected mice. Immunology. 1980 Oct;41(2):387–392. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Mathur A., Mehrotra R. M. Experimentally produced cardiac injury following dengue virus infection. Indian J Pathol Bacteriol. 1974 Oct;17(4):218–220. [PubMed] [Google Scholar]
- Chaturvedi U. C., Shukla M. I., Mathur K. R., Mathur A. Dengue virus-induced cytotoxic factor suppresses immune response of mice to sheep erythrocytes. Immunology. 1981 Jun;43(2):311–316. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Shukla M. I. [Characterization of the suppressor factor produced in the spleen of dengue virus-infected mice]. Ann Immunol (Paris) 1981 May-Jun;132C(3):245–255. doi: 10.1016/0769-2625(81)90075-1. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Tandon H. O., Mathur A. Control of in vitro and in vivo spread of coxsackievirus B4 infection by sensitized spleen cells and antibody. J Infect Dis. 1978 Aug;138(2):181–190. doi: 10.1093/infdis/138.2.181. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Tandon P., Mathur A. Effect of immunosuppression on dengue virus infection in mice. J Gen Virol. 1977 Sep;36(3):449–458. doi: 10.1099/0022-1317-36-3-449. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. Phagocytosis by human monocytes. Blood. 1968 Sep;32(3):423–435. [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- Dalakoti H., Chaturvedi U. C., Mathur A. Inhibition of production of dengue virus induced cytotoxic factor by treatment with cycloheximide & mitomycin C. Indian J Exp Biol. 1982 Jan;20(1):1–3. [PubMed] [Google Scholar]
- Denman A. M., Pinder M. Measruement of immunological function in man: interaction between virus and human leukocytes. Proc R Soc Med. 1974 Dec;67(12 Pt 1):1219–1221. [PMC free article] [PubMed] [Google Scholar]
- Diamantstein T., Ulmer A. Two distinct lumphocyte-stimulating soluble factors (LAF) released from murine peritoneal cells. I. The cellular source and the effect of cGMP on their release. Immunology. 1976 May;30(5):741–747. [PMC free article] [PubMed] [Google Scholar]
- Friedman H. Macrophages in immunity--concluding remarks and general summary. Fed Proc. 1978 Jan;37(1):102–104. [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. Defect in intracellular killing of Staphylococcus aureus within alveolar macrophages in Sendai virus-infected murine lungs. J Clin Invest. 1976 Jun;57(6):1533–1539. doi: 10.1172/JCI108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinerman E. S., Daniels C. A., Polisson R. P., Snyderman R. Effect of virus infection on the inflammatory response. Depression of macrophage accumulation in influenza-infected mice. Am J Pathol. 1976 Nov;85(2):373–382. [PMC free article] [PubMed] [Google Scholar]
- Löhler J., Ehlerding I., Lehmann-Grube F. Pathologie des lymphatischen Gewebes bei der Lymphozytären Choriomeningitis der Maus. Zentralbl Bakteriol Orig A. 1974;227(1-4):458–468. [PubMed] [Google Scholar]
- MIMS C. A. THE PERITONEAL MACROPHAGES OF MICE. Br J Exp Pathol. 1964 Feb;45:37–43. [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979 Mar;43(1):1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayegani M., Lief F. S., Mudd S. Specific and nonspecific cell-mediated resistance to influenza virus in mice. Infect Immun. 1974 Jun;9(6):991–998. doi: 10.1128/iai.9.6.991-998.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla M. I., Chaturvedi U. C. Cycloheximide & mitomycin C inhibited production of dengue virus-induced suppressor factor. Indian J Exp Biol. 1981 Sep;19(9):826–828. [PubMed] [Google Scholar]
- Shukla M. I., Chaturvedi U. C. Dengue virus-induced suppressor factor stimulates production of prostaglandin to mediate suppression. J Gen Virol. 1981 Oct;56(Pt 2):241–249. doi: 10.1099/0022-1317-56-2-241. [DOI] [PubMed] [Google Scholar]
- Tandon P., Chaturvedi U. C., Mathur A. Dengue virus-induced thymus-derived suppressor cells in the spleen of mice. Immunology. 1979 Dec;38(4):653–658. [PMC free article] [PubMed] [Google Scholar]
- Tandon P., Chaturvedi U. C., Mathur A. Differential depletion of T lymphocytes in the spleen of dengue virus-infected mice. Immunology. 1979 May;37(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L. Mechanisms of immunodepression induced by viruses: possible role of infected macrophages. Biomedicine. 1975 Jul;22(4):255–261. [PubMed] [Google Scholar]
- Warshauer D., Goldstein E., Akers T., Lippert W., Kim M. Effect of influenza viral infection on the ingestion and killing of bacteria by alveolar macrophages. Am Rev Respir Dis. 1977 Feb;115(2):269–277. doi: 10.1164/arrd.1977.115.2.269. [DOI] [PubMed] [Google Scholar]
- van Loon A. M., van der Logt J. T., van der Veen J. Poliovirus-induced suppression of lymphocyte stimulation: a macrophage-mediated effect. Immunology. 1979 May;37(1):135–143. [PMC free article] [PubMed] [Google Scholar]


