Abstract
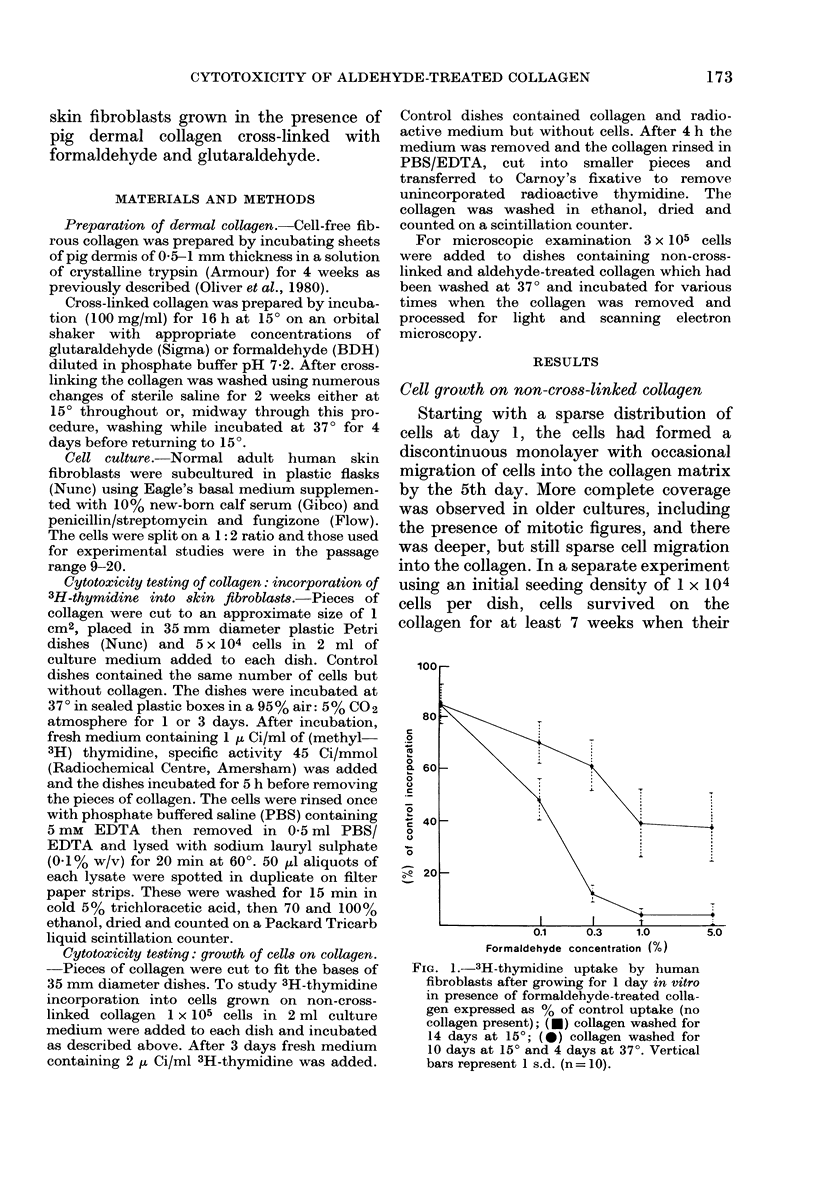
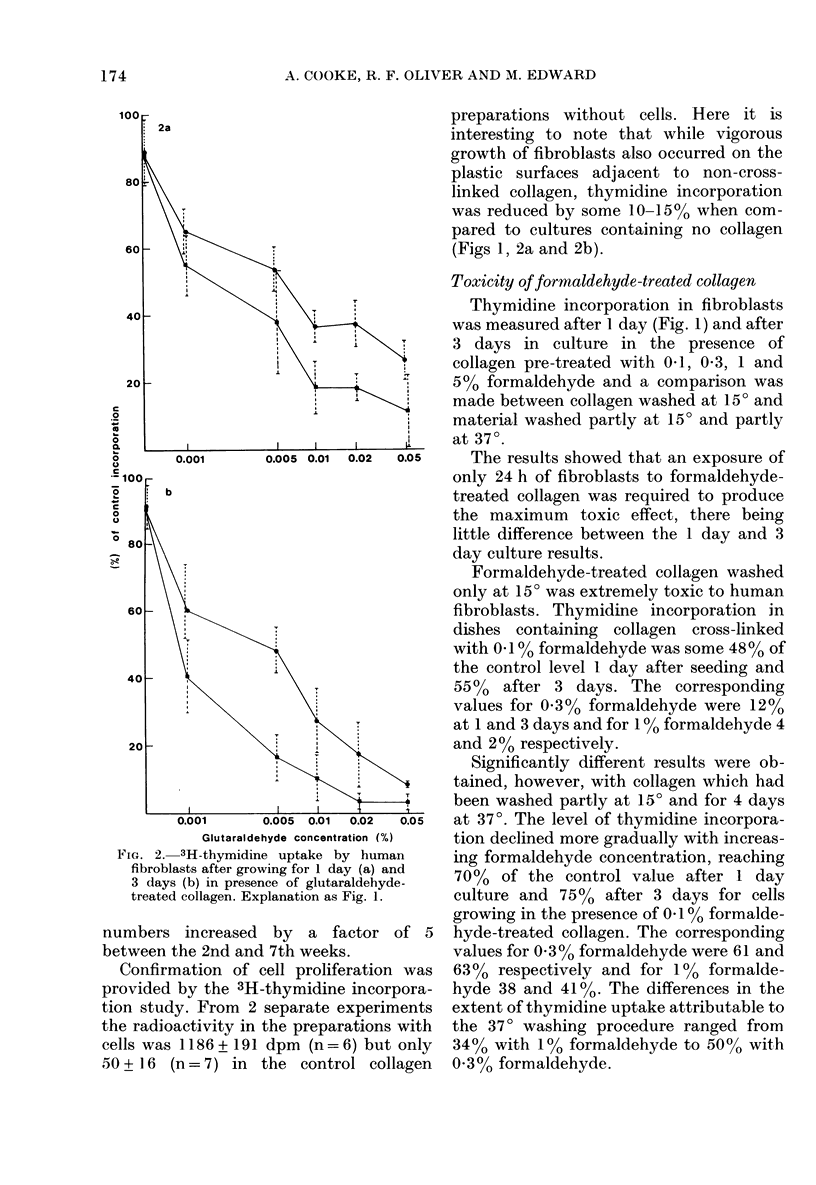
The cytotoxicity of aldehyde-treated collagen was assayed by measuring 3H-thymidine incorporation in adult human skin fibroblasts grown in tissue culture for 1 or 3 days in the presence of pig dermal collagen cross-linked with formaldehyde or glutaraldehyde. A comparison was also made with collagen preparations washed for 2 weeks either at 15 degrees throughout or partly at 15 degrees and partly at 37 degrees. Collagen treated with both formaldehyde and glutaraldehyde proved increasingly toxic with increase in the concentrations of aldehyde used. While the maximum toxic effect was observed after 1 day culture in formaldehyde-treated collagen, with thymidine uptake ranging from 4-48% of control values with 5-0.1% formaldehyde and a 15 degrees wash, the toxic effect of glutaraldehyde treatment increased with longer exposure and at 3 days thymidine uptake ranged from 3-40% of control values with 0.05-0.001% glutaraldehyde and washing at 15 degrees. Washing partly at 37 degrees significantly reduced toxicity, the differences in thymidine uptake as compared with washing at 15 degrees alone ranging from 34-50% with 1 and 0.3% formaldehyde respectively in 1 day cultures and from 14-37% with 0.02 and 0.005% glutaraldehyde in 3 day cultures. While fibroblasts actively grew and migrated when seeded on non-cross-linked collagen, only limited cell survival occurred on aldehyde-treated collagen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker H., Oliver R., Grant R., Stephen L. Formaldehyde as a pre-treatment for dermal collagen heterografts. Biochim Biophys Acta. 1980 Nov 3;632(4):589–597. doi: 10.1016/0304-4165(80)90335-9. [DOI] [PubMed] [Google Scholar]
- Oliver R. F., Barker H., Cooke A., Stephen L. 3H-collagen turnover in non-cross-linked and aldehyde-cross-linked dermal collagen grafts. Br J Exp Pathol. 1982 Feb;63(1):13–17. [PMC free article] [PubMed] [Google Scholar]
- Oliver R. F., Grant R. A., Cox R. W., Cooke A. Effect of aldehyde cross-linking on human dermal collagen implants in the rat. Br J Exp Pathol. 1980 Oct;61(5):544–549. [PMC free article] [PubMed] [Google Scholar]
- Oliver R. F., Grant R. A., Cox R. W., Hulme M. J., Mudie A. Histological studies of subcutaneous and intraperitoneal implants of trypsin-prepared dermal collagen allografts in the rat. Clin Orthop Relat Res. 1976 Mar-Apr;(115):291–302. [PubMed] [Google Scholar]
- Oliver R. F., Grant R. A., Kent C. M. The fate of cutaneously and subcutaneously implanted trypsin purified dermal collagen in the pig. Br J Exp Pathol. 1972 Oct;53(5):540–549. [PMC free article] [PubMed] [Google Scholar]
- Shakespeare P. G., Griffiths R. W. Dermal collagen implants in man. Lancet. 1980 Apr 12;1(8172):795–796. doi: 10.1016/s0140-6736(80)91294-5. [DOI] [PubMed] [Google Scholar]
- Speer D. P., Chvapil M., Eskelson C. D., Ulreich J. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J Biomed Mater Res. 1980 Nov;14(6):753–764. doi: 10.1002/jbm.820140607. [DOI] [PubMed] [Google Scholar]
- Woodroof E. A. Use of glutaraldehyde and formaldehyde to process tissue heart valves. J Bioeng. 1978 Apr;2(1-2):1–9. [PubMed] [Google Scholar]


