Abstract
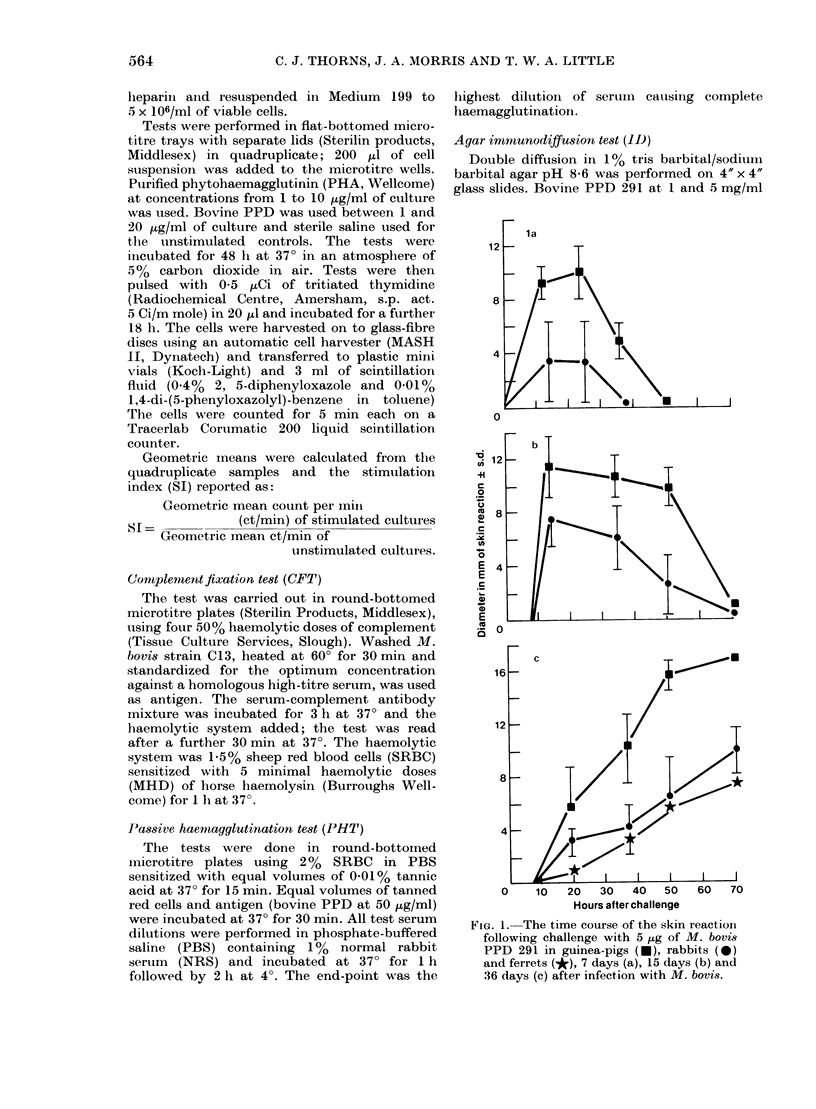
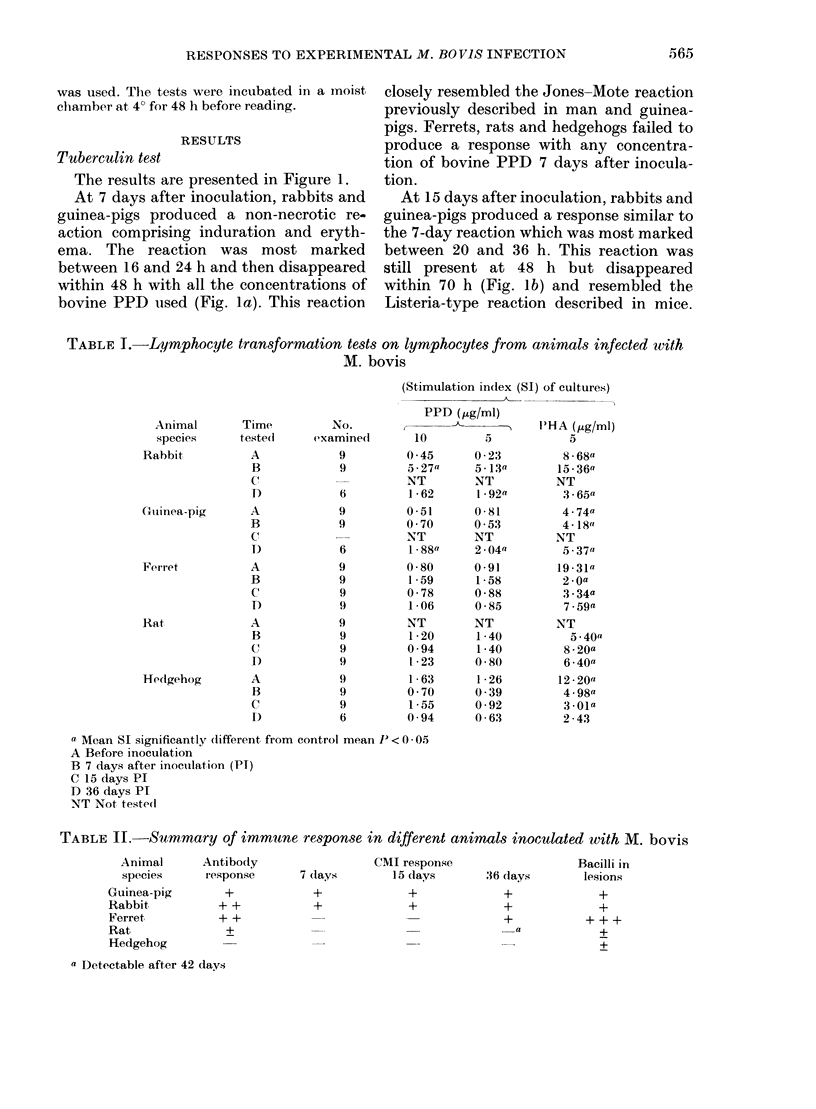
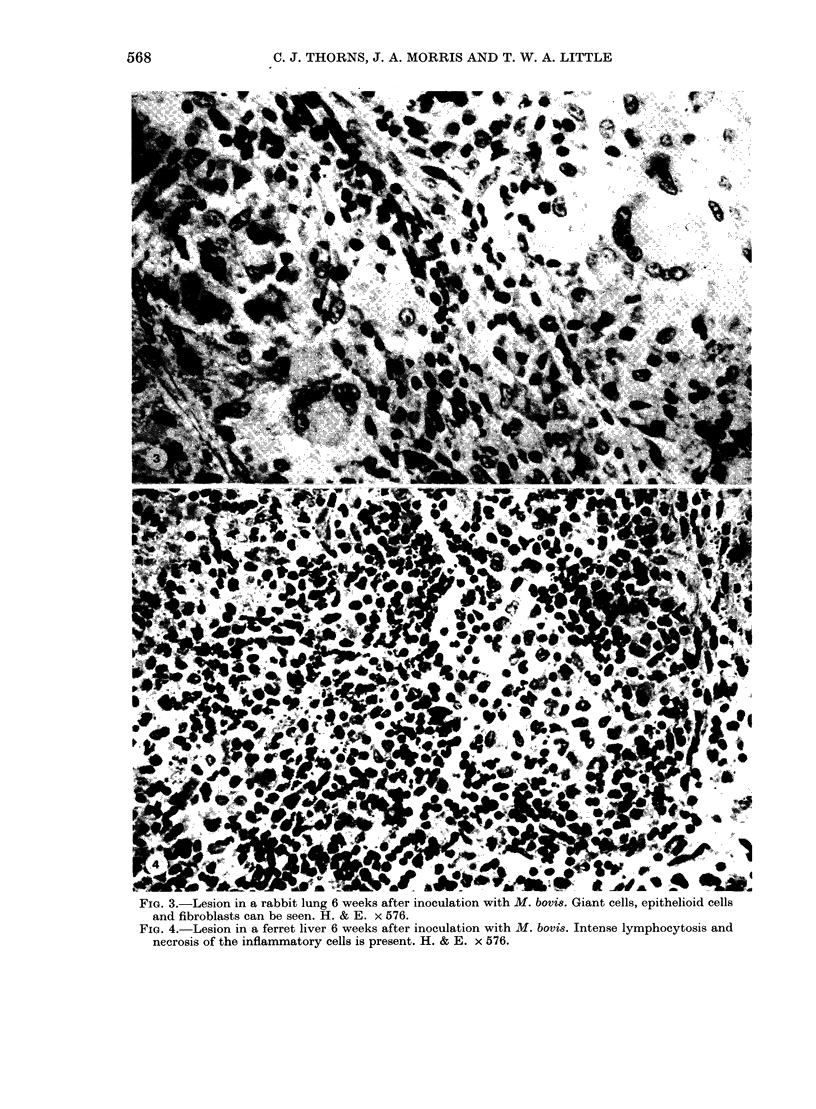
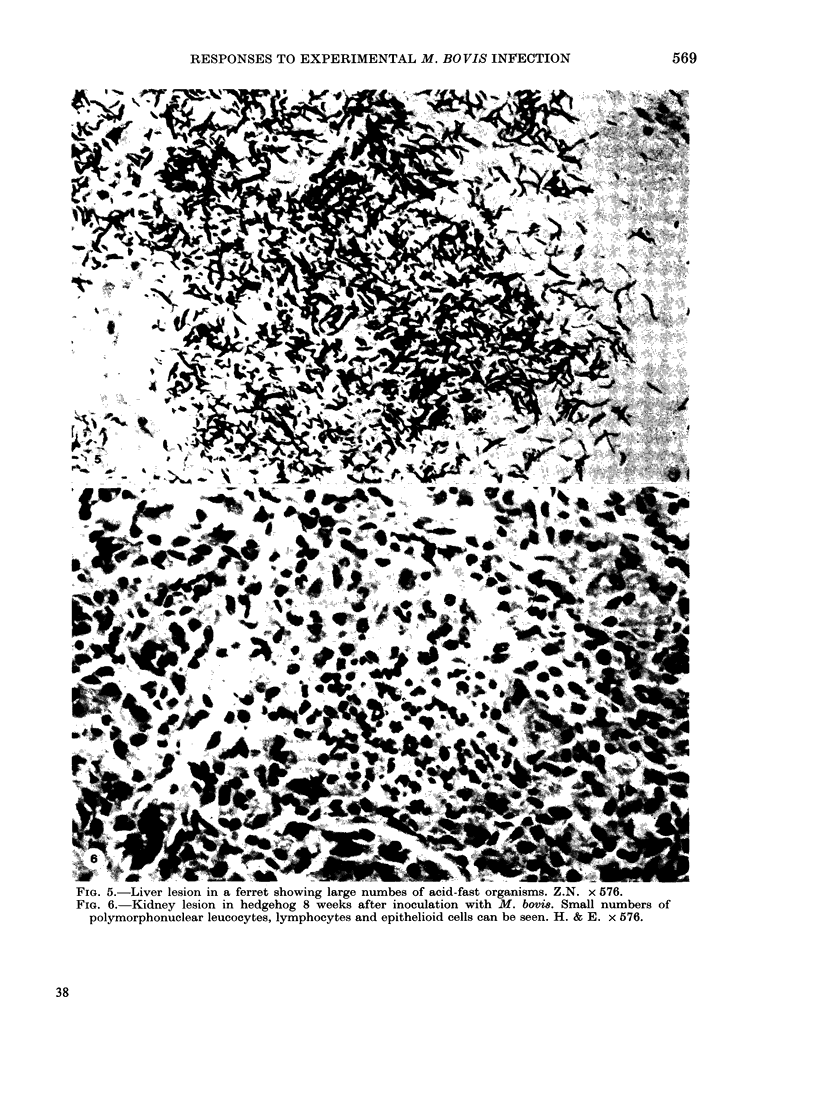
Guinea-pigs, rabbits, rats, ferrets and hedgehogs were infected with a recent field isolate of Mycobacterium bovis. The cell-mediated and antibody responses were studied up to 8 weeks after infection at which time the animals were killed and pathological, histological and bacteriological examinations were carried out. Guinea-pigs and rabbits produced an intense cell-mediated response and strong tissue reactions around the lesions. This appears, in part, to be responsible for the susceptibility of these animals to M. bovis. The strong cell-mediated response was also related to the small numbers of organisms in the tissues. Ferrets produced very little cell-mediated response and only minor tissue reactions. The lack of any cell-mediate response was related to the large numbers of organisms in the tissues which produced an acute disseminated disease. The antibody response produced by ferrets, rabbits and guinea-pigs was variable within an between the species and could not be related to numbers of organisms in the tissues. In rats and hedgehogs a specific cell-mediated and humoral response was difficult to detect but the growth of the organism was controlled by the host resulting in a persistent subclinical infection with no mortality.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brochier J. MLR-R and MLR-S gene products are expressed on different lymphoid cells. Transplantation. 1977 Jul;24(1):63–69. doi: 10.1097/00007890-197707000-00009. [DOI] [PubMed] [Google Scholar]
- Drexhage H. A., Blomberg-vd Flier B. M., vd Berg W. B. Studies on the effect of mycobacterial antibodies on skin-test reactivity to M. tuberculosis. Br J Exp Pathol. 1980 Apr;61(2):186–194. [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Janossy G., Doenhoff M. Activation of human T and B lymphocytes by polyclonal mitogens. Nature. 1974 Apr 19;248(5450):698–701. doi: 10.1038/248698a0. [DOI] [PubMed] [Google Scholar]
- Himeno K., Nomoto K., Kuroiwa A., Miyazaki S., Takeya K. Relation between delayed skin reactivity and macrophage migration inhibition or lymphocyte transformation in tuberculin-type hypersensitivity and Jones-Mote hypersensitivity. Microbiol Immunol. 1977;21(2):99–110. doi: 10.1111/j.1348-0421.1977.tb02812.x. [DOI] [PubMed] [Google Scholar]
- LESSLIE I. W. A comparison of biological and some cultural methods for the primary isolation of Mycobacterium tuberculosis. J Comp Pathol. 1959 Jan;69(1):1–10. doi: 10.1016/s0368-1742(59)80001-1. [DOI] [PubMed] [Google Scholar]
- Lenzini L., Rottoli P., Rottoli L. The spectrum of human tuberculosis. Clin Exp Immunol. 1977 Feb;27(2):230–237. [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The immunology of antituberculous immunity. Am Rev Respir Dis. 1968 Mar;97(3):337–344. doi: 10.1164/arrd.1968.97.3.337. [DOI] [PubMed] [Google Scholar]
- Morris J. A., Stevens A. E., Little T. W., Stuart P. Lymphocyte unresponsiveness to PPD tuberculin in badgers infected with Mycobacterium bovis. Res Vet Sci. 1978 Nov;25(3):390–392. [PubMed] [Google Scholar]
- PEARMAIN G., LYCETTE R. R., FITZGERALD P. H. Tuberculin-induced mitosis in peripheral blood leucocytes. Lancet. 1963 Mar 23;1(7282):637–638. doi: 10.1016/s0140-6736(63)91275-3. [DOI] [PubMed] [Google Scholar]
- PULLING F. B. An outbreak of bovine tuberculosis in mink and treatment with rimifon. J Am Vet Med Assoc. 1952 Nov;121(908):389–390. [PubMed] [Google Scholar]
- Rook G. A., Stanford J. L. The relevance to protection of three forms of delayed skin-test response evoked by m. leprae and other mycobacteria in mice. Correlation with the classical work in the guinea-pig. Parasite Immunol. 1979 Summer;1(2):111–123. doi: 10.1111/j.1365-3024.1979.tb00699.x. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Rosenthal A. S. Peritoneal exudate lymphocyte. 3. Dissociation of antigen-reactive lymphocytes from antigen-binding cells in a T lymphocyte enriched population in the guinea pig. J Immunol. 1974 Mar;112(3):1085–1093. [PubMed] [Google Scholar]
- Roupe G., Strannegård O. The influence of antibody on the induction and elicitation of allergic contact dermatitis. Int Arch Allergy Appl Immunol. 1972;43(5):691–699. doi: 10.1159/000230885. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Oppenheim J. J. Synergistic interaction of macrophages and lymphocytes in antigen-induced transformation of lymphocytes. J Exp Med. 1970 Jul 1;132(1):44–65. doi: 10.1084/jem.132.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen C. O., Richards W. D., Jarnagin J. L. Mycobacteria isolated from exotic animals. J Am Vet Med Assoc. 1977 May 1;170(9):987–990. [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- Weksler M. E., Kuntz M. M. Synergy between human T and B lymphocytes in their response to phythaemagglutinin and pokeweed mitogen. Immunology. 1976 Aug;31(2):273–281. [PMC free article] [PubMed] [Google Scholar]






