Abstract
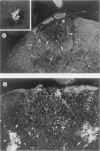
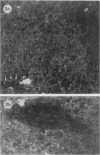
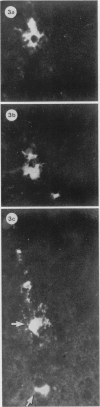
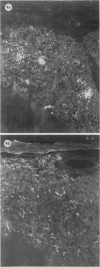
The present study has shown that virus can be readily detected by immunofluorescent staining in the central nervous system (CNS) of SJL mice persistently infected with Theiler's murine encephalomyelitis virus (TMEV). Considering the low CNS virus content, large amounts of virus antigen were found in the white matter, the site of demyelinating lesions. Virus antigen was detected in all animals killed after post-infection (PI) Day 21, a time which can be considered as the beginning of the persistent phase of this infection, and the appearance of virus antigen in white matter corresponded closely in time with the onset of demyelination. The pathogensis of this persistent infection can now be reasonably well reconstructed from the temporal observations made in this study. It would appear that between the second and third week PI, virus replication largely shifts from neurons in spinal cord gray matter to other cell types located in white matter. While a lower-grade persistent infection (in terms of the relative number of cells containing virus antigen) is established and maintained in cells in the gray matter and inflammatory and leptomeningeal infiltrates, cells in white matter appear to be mainly responsible for perpetuating the infection. Why these cells should supplant neurons as the most susceptible host cell during the chronic phase of the infection is discussed.
Full text
PDF

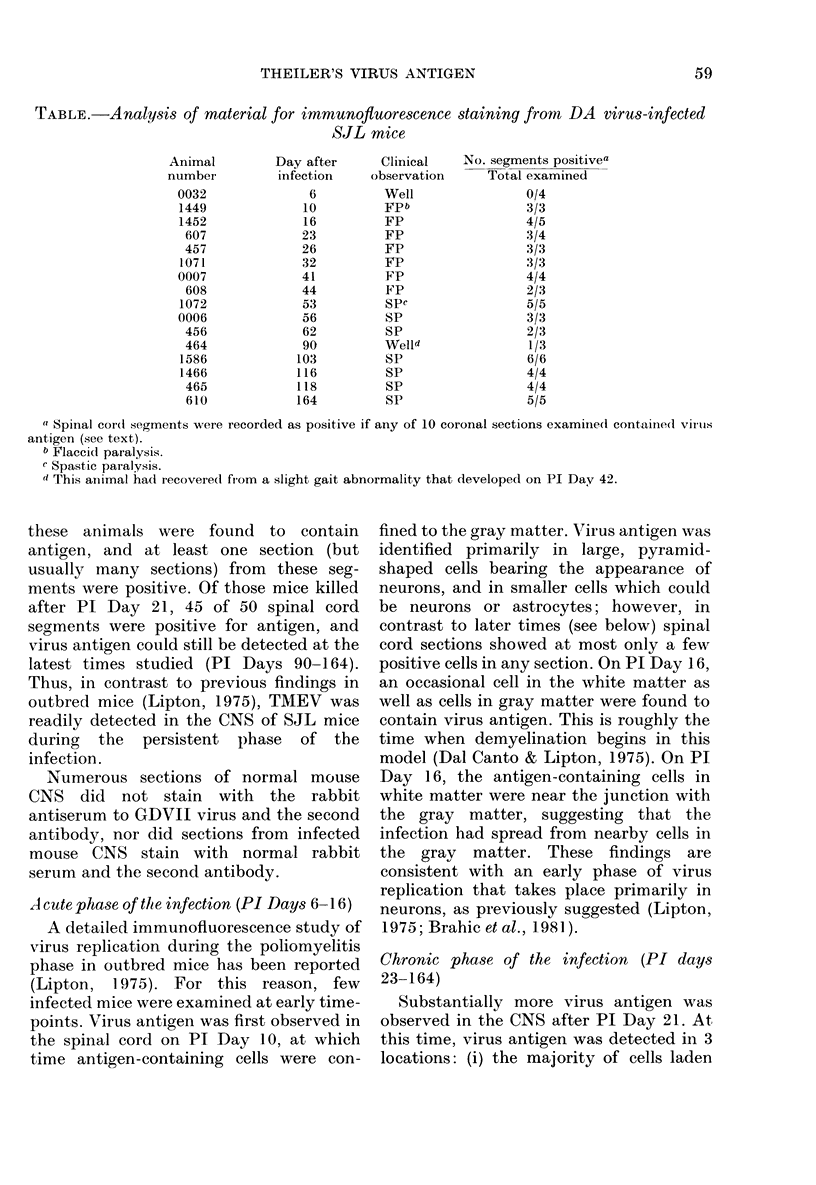
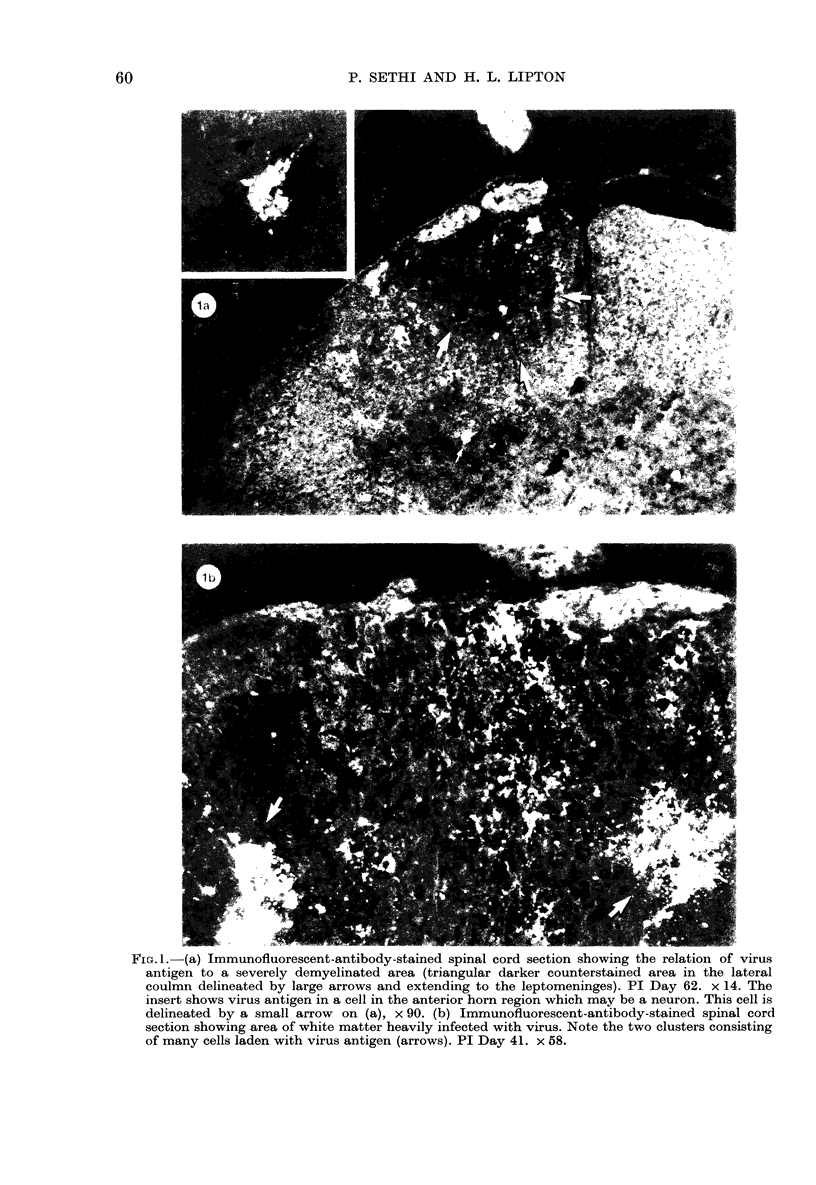
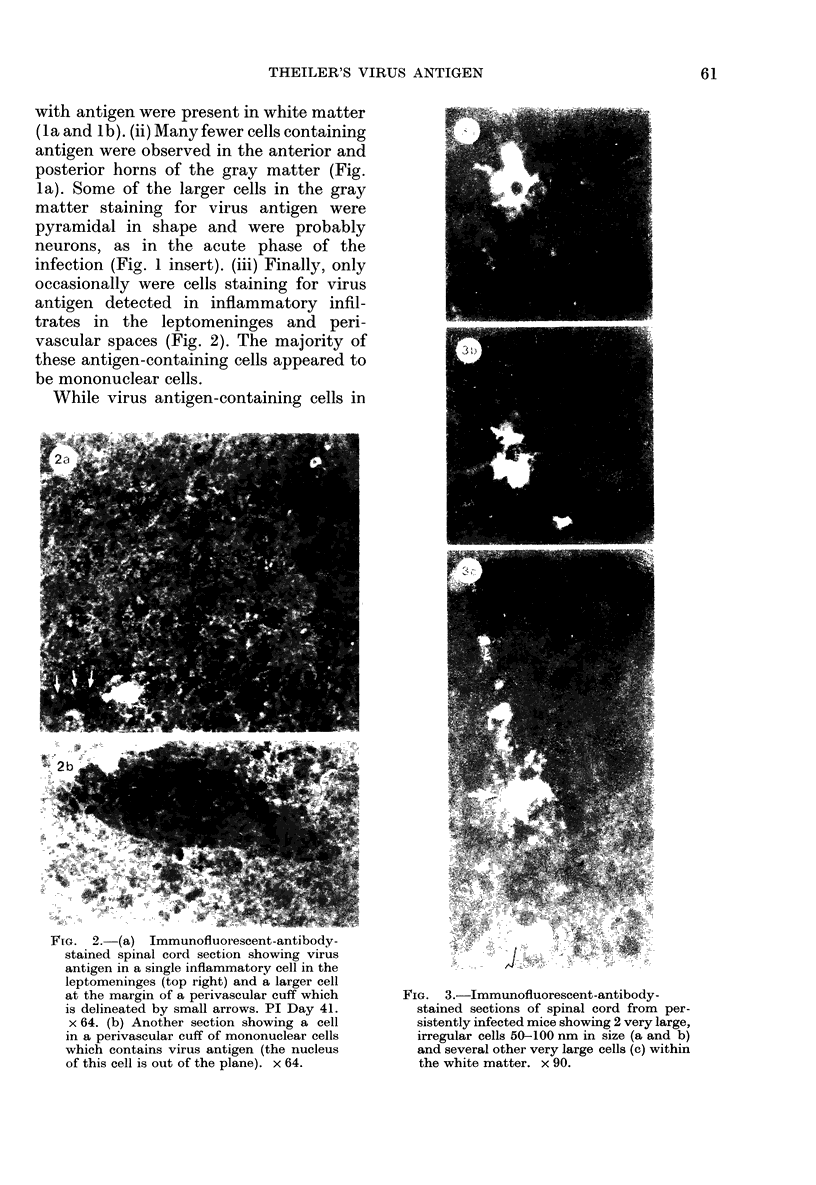



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brahic M., Stroop W. G., Baringer J. R. Theiler's virus persists in glial cells during demyelinating disease. Cell. 1981 Oct;26(1 Pt 1):123–128. doi: 10.1016/0092-8674(81)90040-4. [DOI] [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Primary demyelination in Theiler's virus infection. An ultrastructural study. Lab Invest. 1975 Dec;33(6):626–637. [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Schwann cell remyelination and recurrent demyelination in the central nervous system of mice infected with attenuated Theiler's virus. Am J Pathol. 1980 Jan;98(1):101–122. [PMC free article] [PubMed] [Google Scholar]
- Friedmann A., Lipton H. L. Replication of Theiler's murine encephalomyelitis viruses in BHK21 cells: an electron microscopic study. Virology. 1980 Mar;101(2):389–398. doi: 10.1016/0042-6822(80)90452-3. [DOI] [PubMed] [Google Scholar]
- Kliks S. C., Halstead S. B. An explanation for enhanced virus plaque formation in chick embryo cells. Nature. 1980 Jun 12;285(5765):504–505. doi: 10.1038/285504a0. [DOI] [PubMed] [Google Scholar]
- Lehrich J. R., Arnason B. G., Hochberg F. H. Demyelinative myelopathy in mice induced by the DA virus. J Neurol Sci. 1976 Oct;29(2-4):149–160. doi: 10.1016/0022-510x(76)90167-2. [DOI] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. Susceptibility of inbred mice to chronic central nervous system infection by Theiler's murine encephalomyelitis virus. Infect Immun. 1979 Oct;26(1):369–374. doi: 10.1128/iai.26.1.369-374.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L. Persistent Theiler's murine encephalomyelitis virus infection in mice depends on plaque size. J Gen Virol. 1980 Jan;46(1):169–177. doi: 10.1099/0022-1317-46-1-169. [DOI] [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B. I., Joseph B. S., Walsh M. J., Potvin A. R., Tourtellotte W. W. Multiple sclerosis serum and cerebrospinal fluid immunoglobulin binding to Fc receptors of oligodendrocytes. Ann Neurol. 1981 Apr;9(4):371–377. doi: 10.1002/ana.410090410. [DOI] [PubMed] [Google Scholar]
- Peiris J. S., Porterfield J. S. Antibody-dependent enhancement of plaque formation on cell lines of macrophage origin - a sensitive assay for antiviral antibody. J Gen Virol. 1981 Nov;57(Pt 1):119–125. doi: 10.1099/0022-1317-57-1-119. [DOI] [PubMed] [Google Scholar]
- Peiris J. S., Porterfield J. S. Antibody-mediated enhancement of Flavivirus replication in macrophage-like cell lines. Nature. 1979 Nov 29;282(5738):509–511. doi: 10.1038/282509a0. [DOI] [PubMed] [Google Scholar]
- Penney J. B., Jr, Wolinsky J. S. Neuronal and oligodendroglial infection by the WW strain of Theiler's virus. Lab Invest. 1979 Mar;40(3):324–330. [PubMed] [Google Scholar]
- Rabinowitz S. G., Lipton H. L. Cellular immunity in chronic Theiler's virus central nervous system infection. J Immunol. 1976 Aug;117(2):357–363. [PubMed] [Google Scholar]
- Roos R. P., Firestone S., Wollmann R., Variakojis D., Arnason B. G. The effect of short-term and chronic immunosuppression on Theiler's virus demyelination. J Neuroimmunol. 1982 Jun;2(3-4):223–234. doi: 10.1016/0165-5728(82)90057-1. [DOI] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W. Growth of 17D yellow fever virus in a macrophage-like cell line, U937: role of Fc and viral receptors in antibody-mediated infection. J Immunol. 1981 Aug;127(2):659–665. [PubMed] [Google Scholar]