Abstract
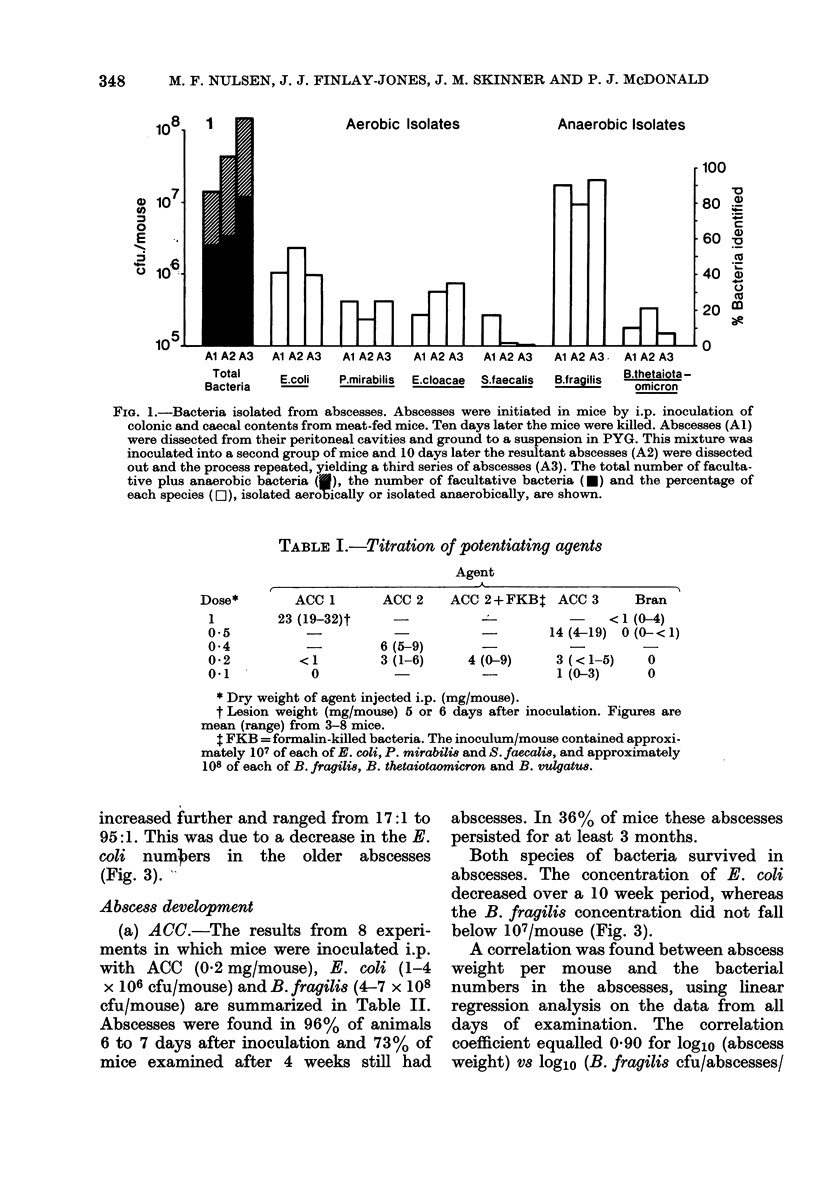
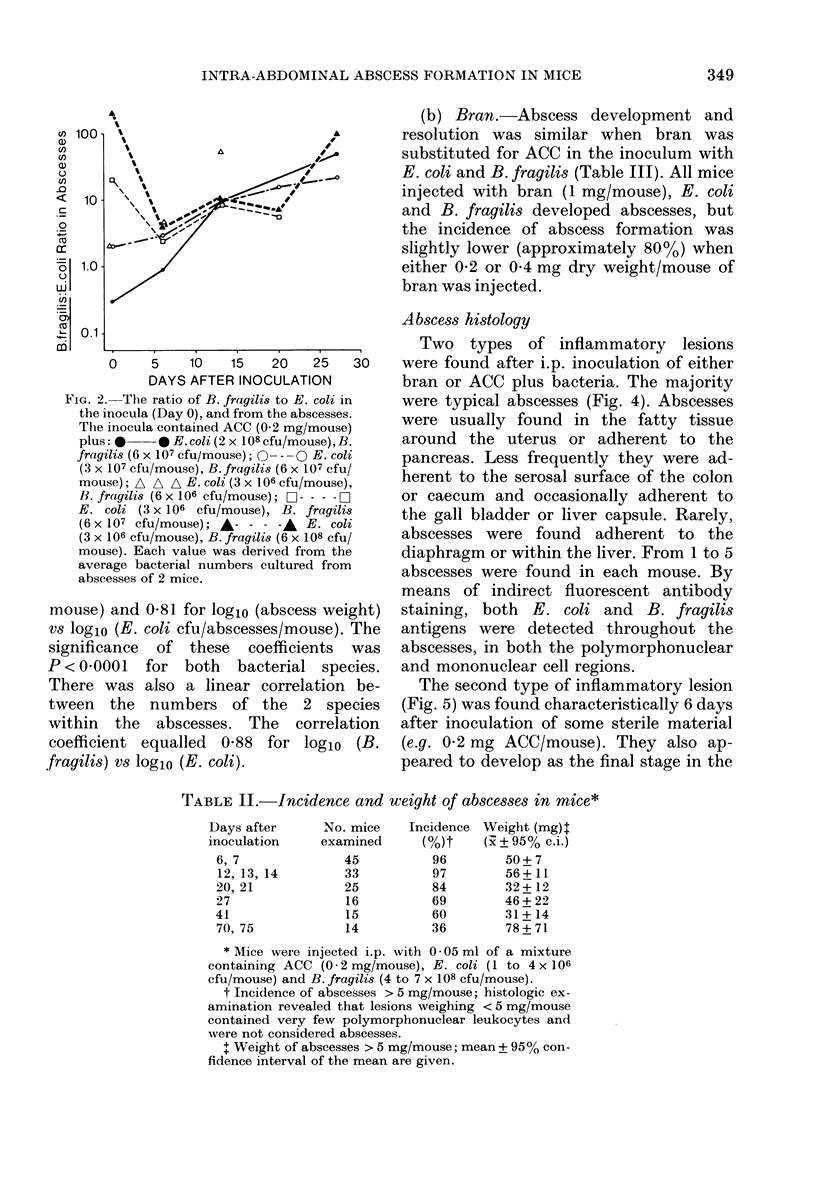
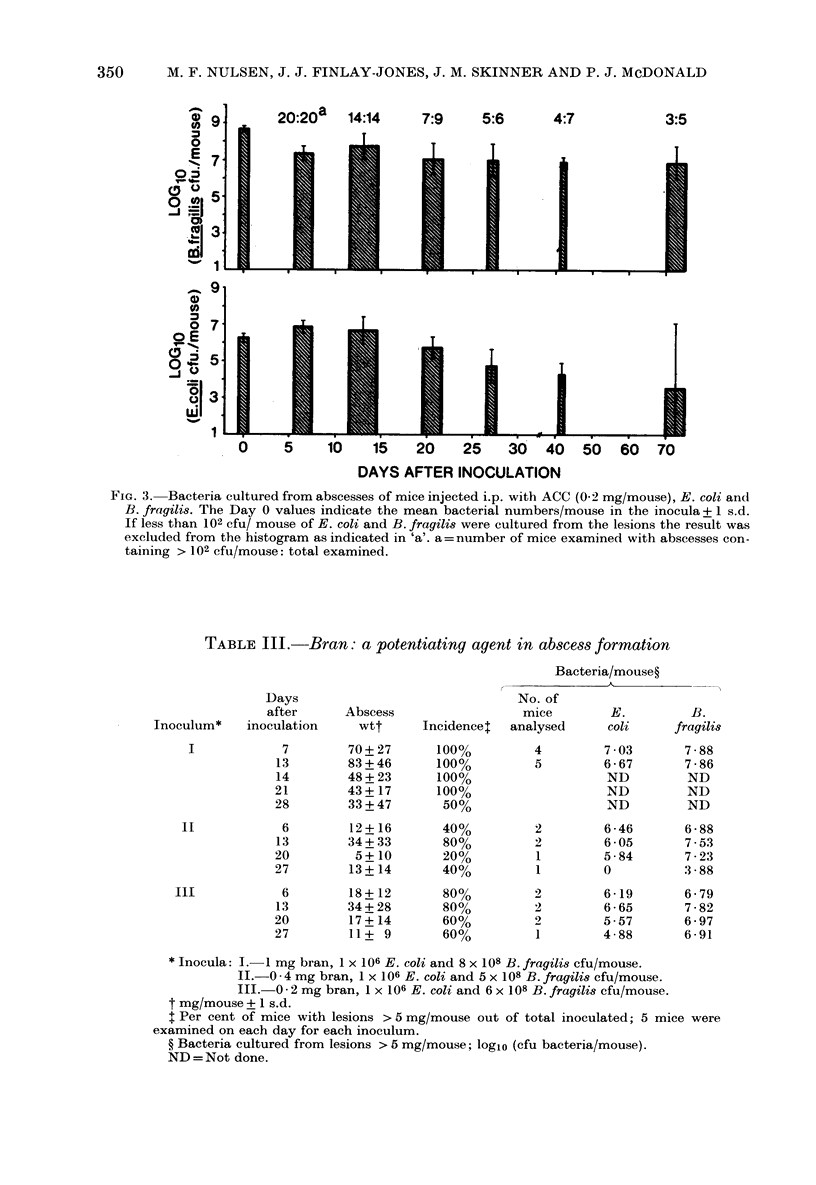
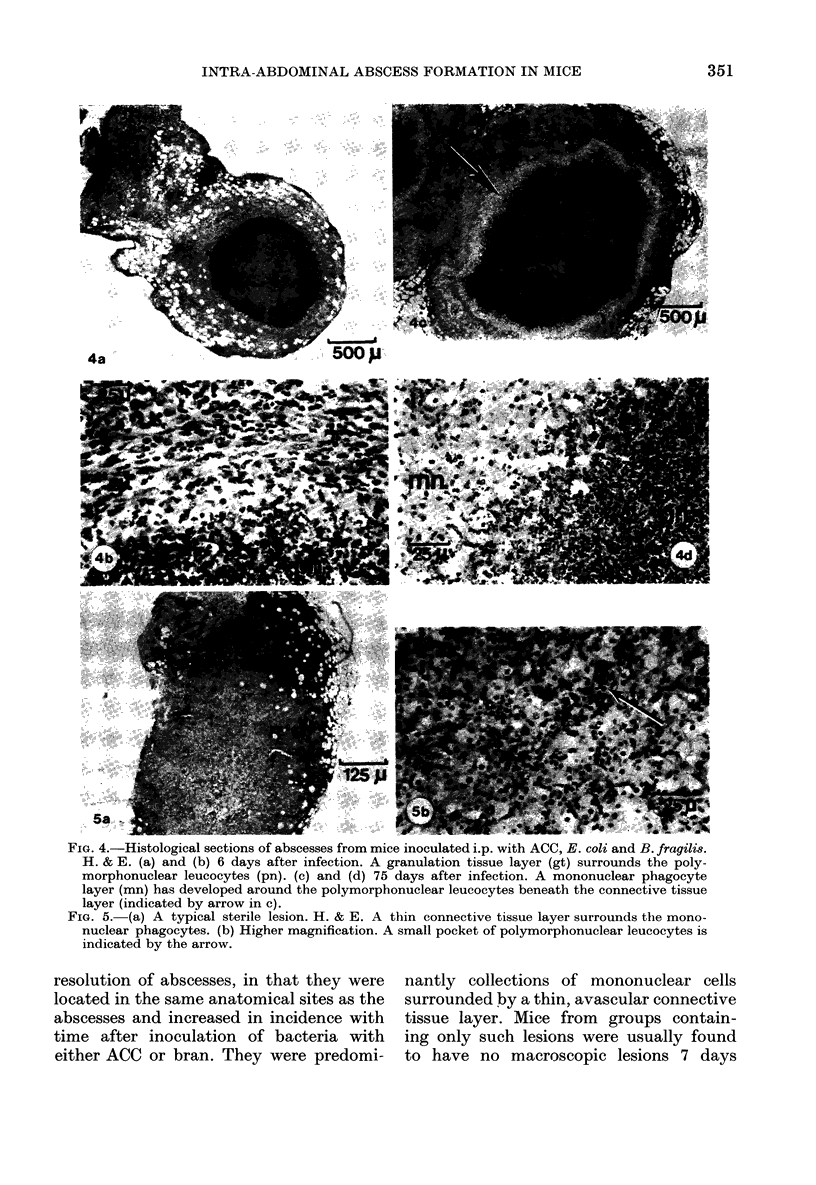
A model of intra-abdominal (IA) abscess formation has been developed in mice. Intraperitoneal (i.p.) injection of a mixture of a potentiating agent (autoclaved colonic and caecal contents (ACC), 0.2 mg dry wt/mouse or sterile bran, 1 mg dry wt/mouse), Escherichia coli (1 X 10(6) colony forming units (cfu)/mouse) and Bacteroides fragilis (5 X 10(8) cfu/mouse) induced abscesses in 98% of mice inoculated. The abscesses persisted for at least 4 weeks in 60% of inoculated animals, and for 10 weeks in 36%. From 1 to 5 abscesses per mouse were found. Abscess formation was quantified by weighing the dissected abscesses and by culturing bacteria from them. Histologically, the abscesses were characterized by a central region of polymorphonuclear leucocytes, often with a thin mononuclear phagocyte infiltrate surrounding it, and an outer wall of vascularized connective tissue. Fluorescent antibody studies demonstrated that antigens from both bacterial species were distributed throughout the abscess. At the concentrations used, neither ACC nor sterile bran induced formation in the absence of viable bacteria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altemeier W. A., Culbertson W. R., Fullen W. D., Shook C. D. Intra-abdominal abscesses. Am J Surg. 1973 Jan;125(1):70–79. doi: 10.1016/0002-9610(73)90010-x. [DOI] [PubMed] [Google Scholar]
- Finegold S. M. Anaerobic infections. Surg Clin North Am. 1980 Feb;60(1):49–64. doi: 10.1016/s0039-6109(16)42033-5. [DOI] [PubMed] [Google Scholar]
- Harris M. A., Reddy C. A., Carter G. R. Anaerobic bacteria from the large intestine of mice. Appl Environ Microbiol. 1976 Jun;31(6):907–912. doi: 10.1128/aem.31.6.907-912.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Onderdonk A. B., Gelfand J. A., Bartlett J. G., Gorbach S. L. A quantitative model for subcutaneous abscess formation in mice. Br J Exp Pathol. 1980 Feb;61(1):97–107. [PMC free article] [PubMed] [Google Scholar]
- Kapral F. A., Godwin J. R., Dye E. S. Formation of intraperitoneal abscesses by Staphylococcus aureus. Infect Immun. 1980 Oct;30(1):204–211. doi: 10.1128/iai.30.1.204-211.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. J. The quantitative and histological demonstration of pathogenic synergy between Escherichia coli and Bacteroides fragilis in guinea-pig wounds. J Med Microbiol. 1978 Nov;11(4):513–523. doi: 10.1099/00222615-11-4-513. [DOI] [PubMed] [Google Scholar]
- McConville J. H., Snyder M. J., Calia F. M., Hornick R. B. Model of intraabdominal abscess in mice. Infect Immun. 1981 Jan;31(1):507–509. doi: 10.1128/iai.31.1.507-509.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan K., Gorbach S. L. Anaerobes in mixed infections. J Infect Dis. 1981 Aug;144(2):181–186. doi: 10.1093/infdis/144.2.181. [DOI] [PubMed] [Google Scholar]
- Mera S. L., Young E. W., Bradfield J. W. Direct immunofluorescence of skin using formalin-fixed paraffin-embedded sections. J Clin Pathol. 1980 Apr;33(4):365–369. doi: 10.1136/jcp.33.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols R. L. Infections following gastrointestinal surgery: intra-abdominal abscess. Surg Clin North Am. 1980 Feb;60(1):197–212. doi: 10.1016/s0039-6109(16)42044-x. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Bartlett J. G., Louie T., Sullivan-Seigler N., Gorbach S. L. Microbial synergy in experimental intra-abdominal abscess. Infect Immun. 1976 Jan;13(1):22–26. doi: 10.1128/iai.13.1.22-26.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Kasper D. L., Cisneros R. L., Bartlett J. G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977 Jul;136(1):82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Kasper D. L., Mansheim B. J., Louie T. J., Gorbach S. L., Bartlett J. G. Experimental animal models for anaerobic infections. Rev Infect Dis. 1979 Mar-Apr;1(2):291–301. doi: 10.1093/clinids/1.2.291. [DOI] [PubMed] [Google Scholar]
- Savage D. C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Tannock G. W. Characteristics of Bacteroides isolates from the cecum of conventional mice. Appl Environ Microbiol. 1977 Apr;33(4):745–750. doi: 10.1128/aem.33.4.745-750.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. B., Wilkins T. D. Use of semisolid agar from initiation of pure Bacteroides fragilis infection in mice. Infect Immun. 1976 Sep;14(3):721–725. doi: 10.1128/iai.14.3.721-725.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]




