Abstract
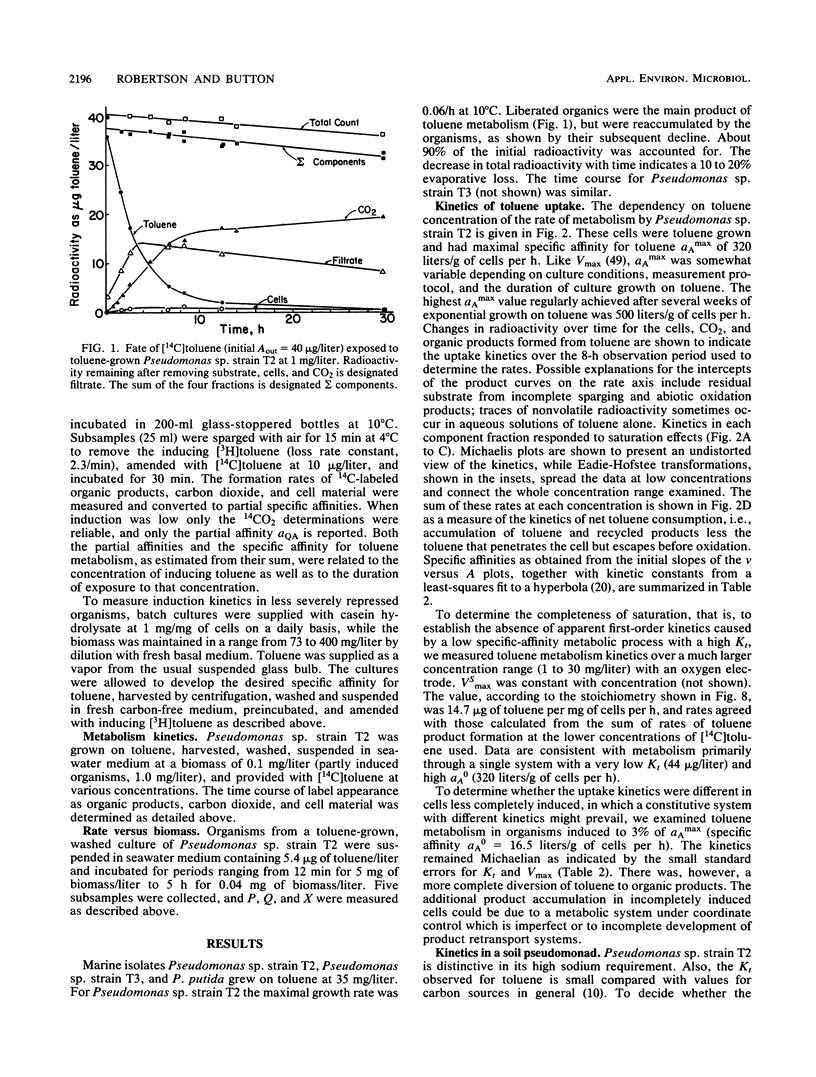
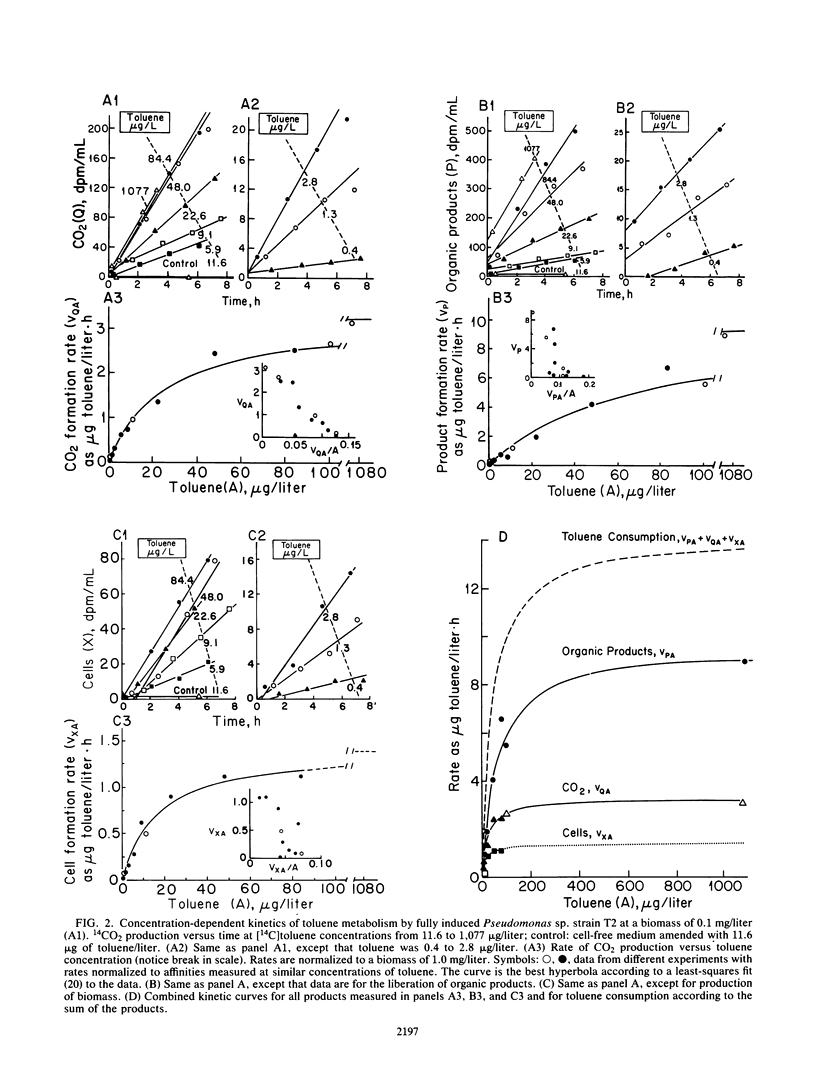
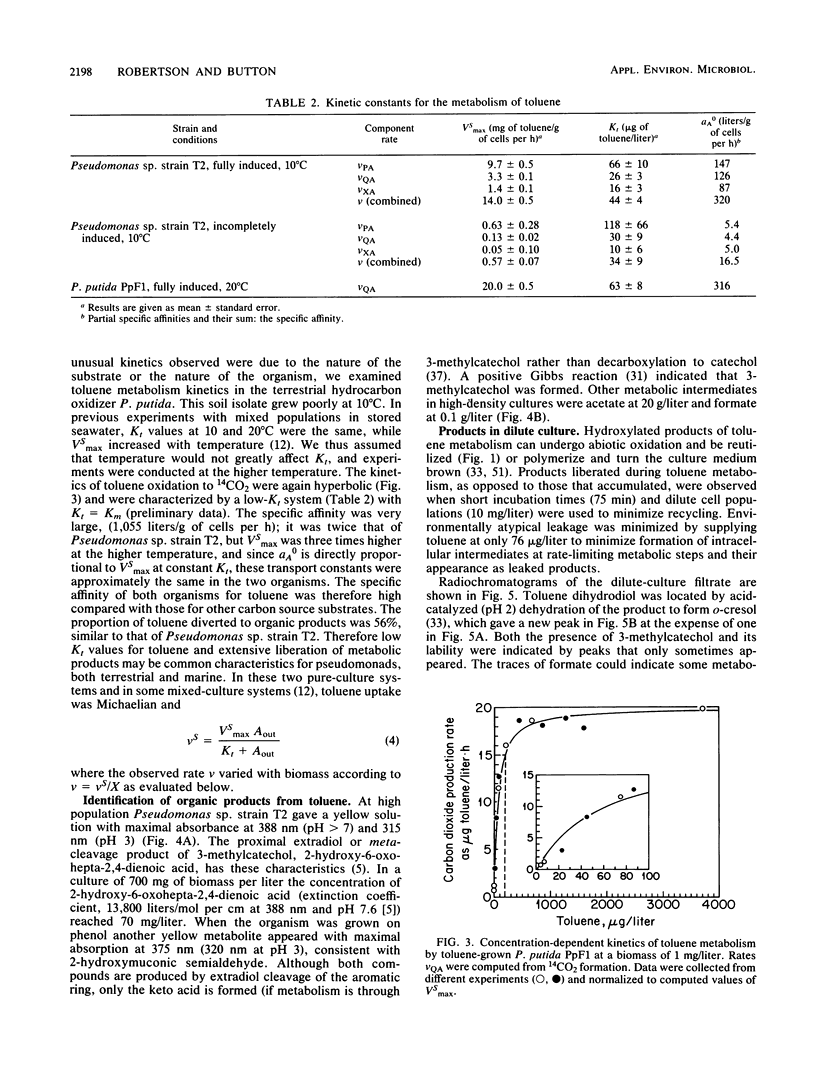
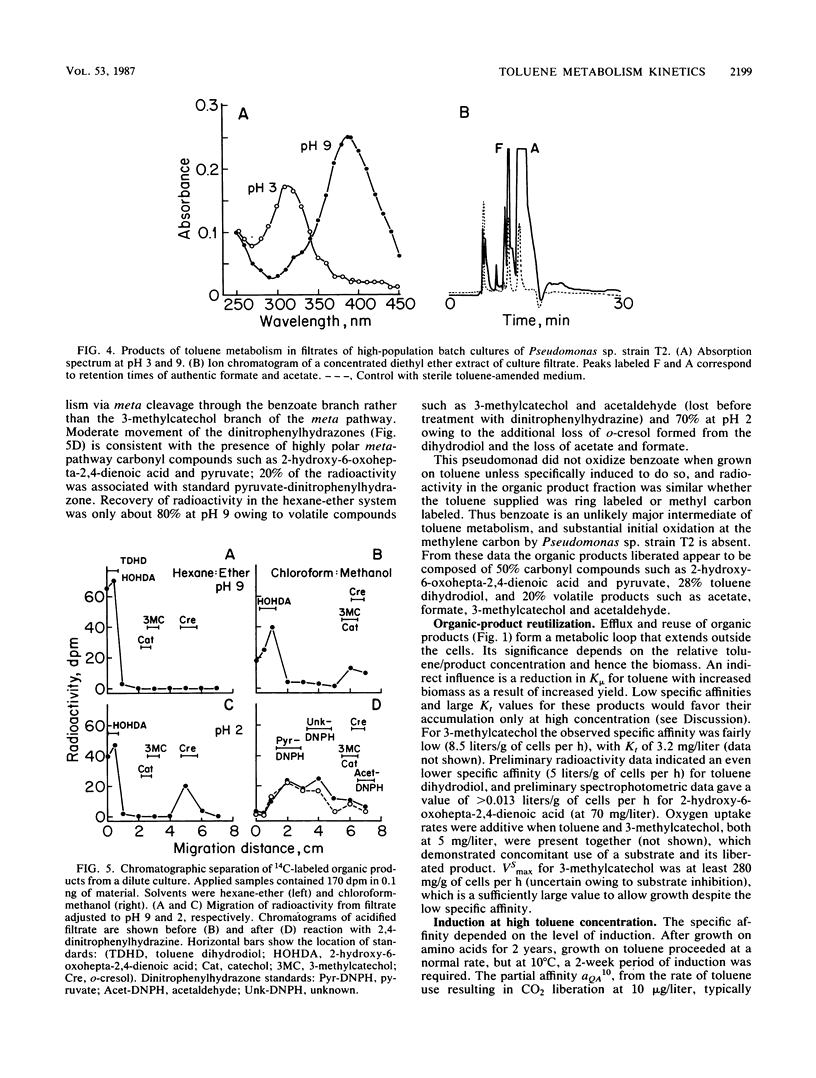
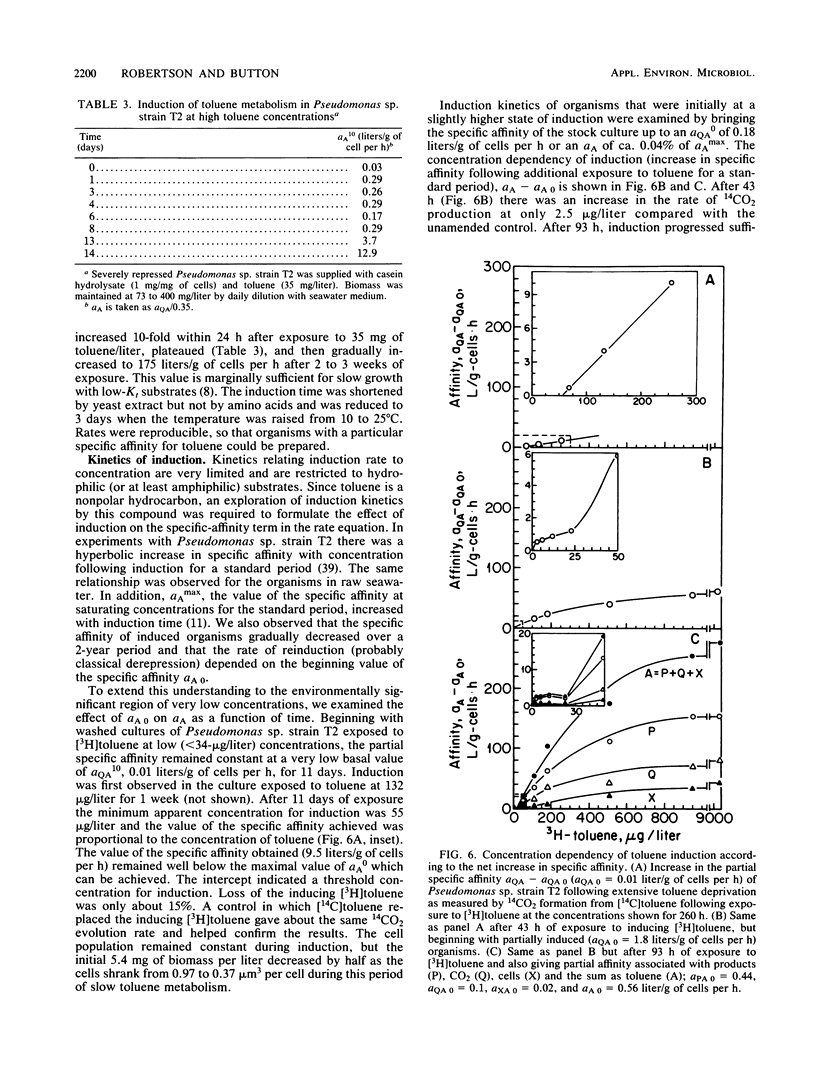
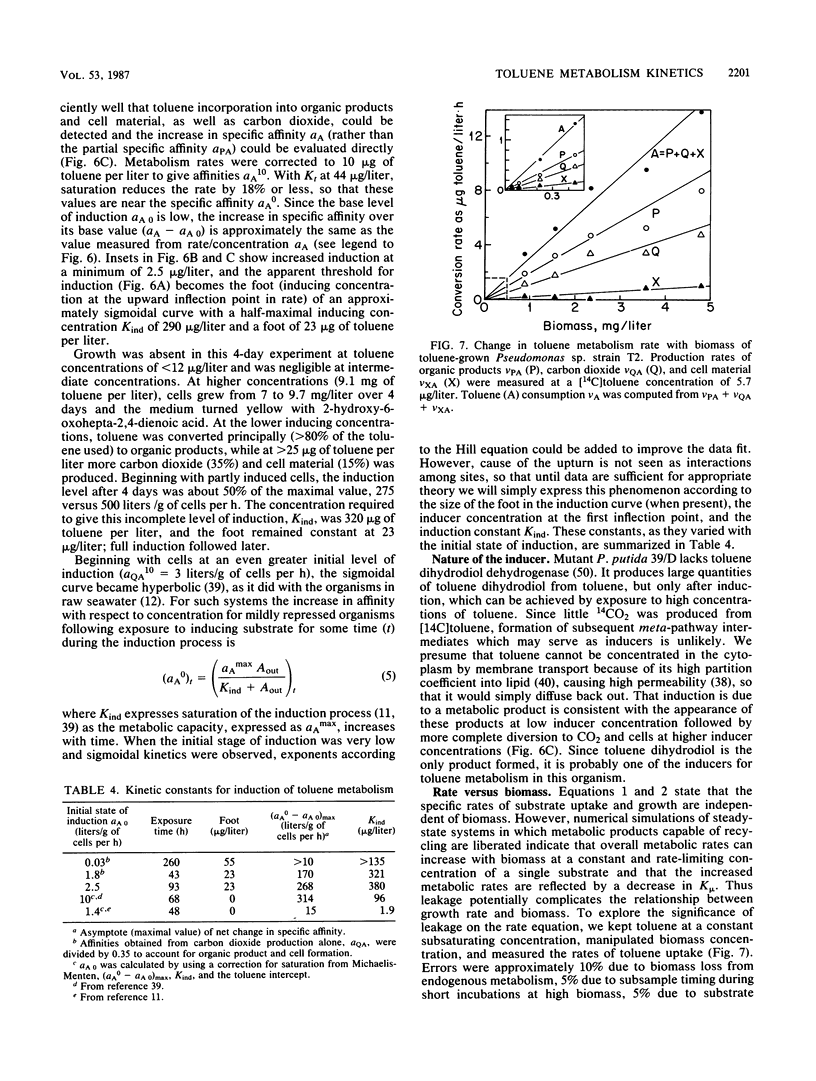
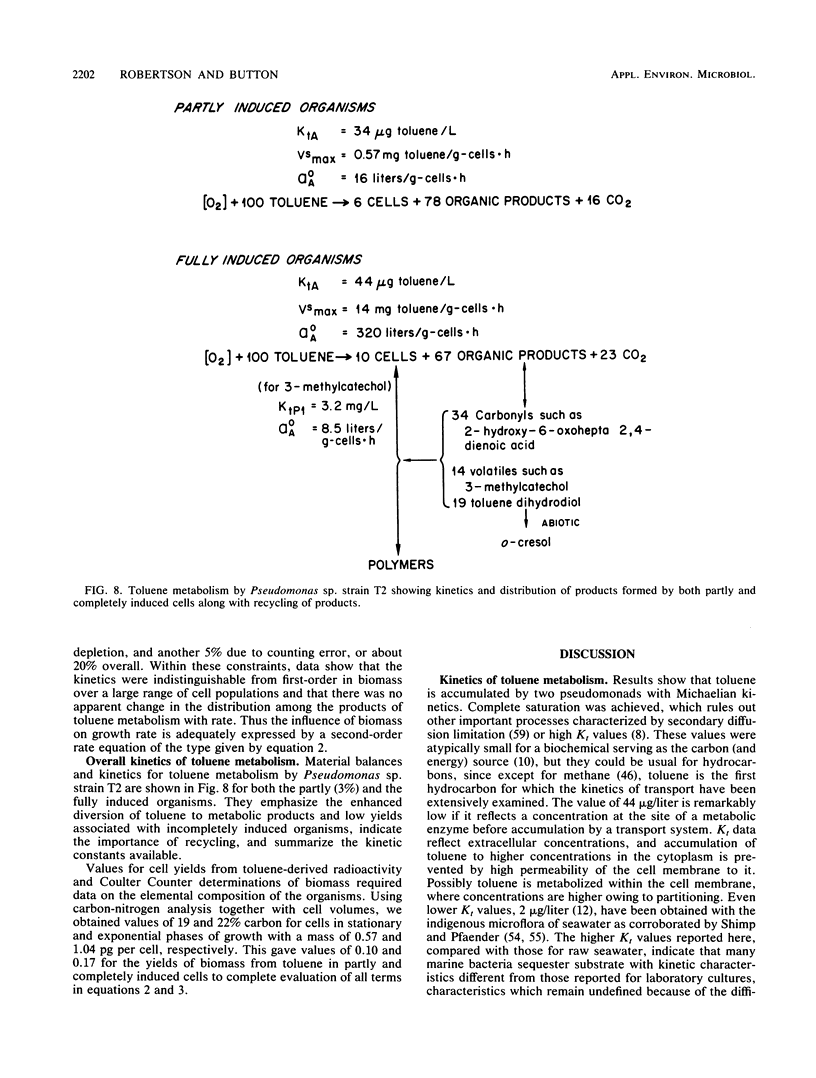
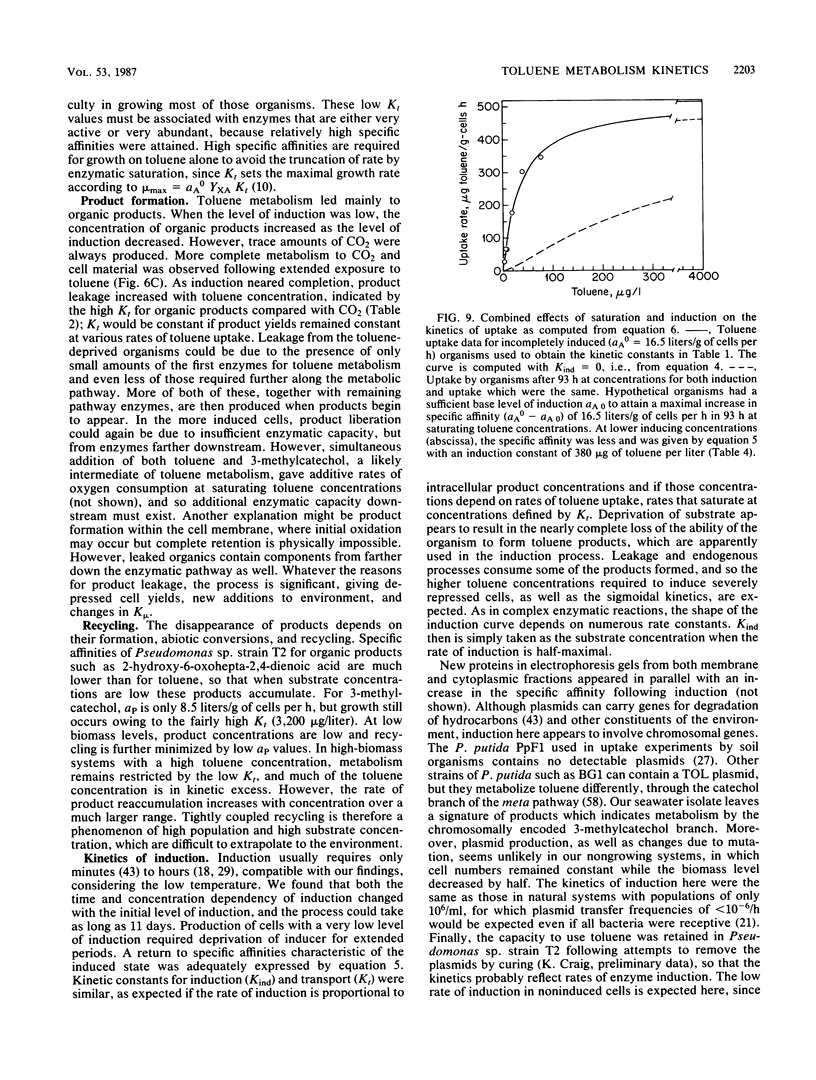
The kinetics of concentration-dependent toluene metabolism were examined by evaluating each term in the second-order rate equation. Marine and freshwater pseudomonads were used. Uptake for Pseudomonas sp. strain T2 was characterized by a completely saturatable system with small transport constant (Kt = 44 μg/liter) and large specific affinity. Kinetics for Pseudomonas putida PpF1 were similar. Induction had little effect on Kt, but it caused the specific affinity to increase from about 0.03 to 320 liters/g of cells per h. The level of induction depended on the time of exposure, the concentration of inducer, and the initial level of induction. If loss of the inducible system was not severe, toluene caused a linear increase in specific affinity with time, and the maximal value achieved at intermediate times (1 to 3 days) was hyperbolic with concentration when Kind was 96 μg/liter (A. T. Law and D. K. Button, Appl. Environ. Microbiol. 51:469-476, 1986). As repression became complete, specific affinities were greatly reduced. Then induction required higher toluene concentrations and longer times, and the shape of the specific-affinity curve became sigmoidal with concentration. Cell yields (0.10 to 0.17 g of cells per g of toluene used) were low owing to liberation of organic products: 2-hydroxy-6-oxohepta-2,4-dienoic acid, toluene dihydrodiol, 3-methylcatechol, acetate, formate, and possibly pyruvate, which in turn caused lower rates of growth. Michaelis constants for the reaccumulation of products exceeded those for toluene, but specific affinities were lower and maximal velocities were higher, so that recycling was favored in cultures with high toluene concentration. Although these kinetics predict deviation from the linear relationship between uptake rate and biomass, we could detect none. Effects of saturation and induction were incorporated into the basic specific-affinity relationship. The result appears to be an improvement in the equation used for describing the kinetics of uptake and growth.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott B. J., Gledhill W. E. The extracellular accumulation of metabolic products by hydrocarbon-degrading microorganisms. Adv Appl Microbiol. 1971;14:249–388. doi: 10.1016/s0065-2164(08)70546-x. [DOI] [PubMed] [Google Scholar]
- Alexander M. Biodegradation of chemicals of environmental concern. Science. 1981 Jan 9;211(4478):132–138. doi: 10.1126/science.7444456. [DOI] [PubMed] [Google Scholar]
- BUTTIN G., COHEN G. N., MONOD J., RICKENBERG H. V. La galactoside-perméase d'Escherichia coli. Ann Inst Pasteur (Paris) 1956 Dec;91(6):829–857. [PubMed] [Google Scholar]
- Barnsley E. A. Role and regulation of the ortho and meta pathways of catechol metabolism in pseudomonads metabolizing naphthalene and salicylate. J Bacteriol. 1976 Feb;125(2):404–408. doi: 10.1128/jb.125.2.404-408.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnsley E. A. The induction of the enzymes of naphthalene metabolism in pseudomonads by salicylate and 2-aminobenzoate. J Gen Microbiol. 1975 May;88(1):193–196. doi: 10.1099/00221287-88-1-193. [DOI] [PubMed] [Google Scholar]
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. A. Local anesthetics block induction of the Pseudomonas alk regulon. J Bacteriol. 1979 Dec;140(3):1123–1125. doi: 10.1128/jb.140.3.1123-1125.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S., Fennewald M., Shapiro J., Huettner C. Fractionation of inducible alkane hydroxylase activity in Pseudomonas putida and characterization of hydroxylase-negative plasmid mutations. J Bacteriol. 1977 Nov;132(2):614–621. doi: 10.1128/jb.132.2.614-621.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K. Evidence for a terpene-based food chain in the gulf of alaska. Appl Environ Microbiol. 1984 Nov;48(5):1004–1011. doi: 10.1128/aem.48.5.1004-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K. Kinetics of nutrient-limited transport and microbial growth. Microbiol Rev. 1985 Sep;49(3):270–297. doi: 10.1128/mr.49.3.270-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K., Robertson B. R., Craig K. S. Dissolved hydrocarbons and related microflora in a fjordal seaport: sources, sinks, concentrations, and kinetics. Appl Environ Microbiol. 1981 Oct;42(4):708–719. doi: 10.1128/aem.42.4.708-719.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K., Schell D. M., Robertson B. R. Sensitive and accurate methodology for measuring the kinetics of concentration-dependent hydrocarbon metabolism rates in seawater by microbial communities. Appl Environ Microbiol. 1981 Apr;41(4):936–941. doi: 10.1128/aem.41.4.936-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAUS D., WALKER N. THE DECOMPOSITION OF TOLUENE BY SOIL BACTERIA. J Gen Microbiol. 1964 Jul;36:107–122. doi: 10.1099/00221287-36-1-107. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Freeman J. P., Mitchum R. K. Glucuronide and sulfate conjugation in the fungal metabolism of aromatic hydrocarbons. Appl Environ Microbiol. 1982 May;43(5):1070–1075. doi: 10.1128/aem.43.5.1070-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Gibson D. T. Metabolism of naphthalene by Cunninghamella elegans. Appl Environ Microbiol. 1977 Oct;34(4):363–370. doi: 10.1128/aem.34.4.363-370.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. H. Adaptation. The Fifteenth Marjory Stephenson Memorial Lecture. J Gen Microbiol. 1981 Sep;126(1):5–20. doi: 10.1099/00221287-126-1-5. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Cullum J., Collins J. F., Broda P. Factors affecting the kinetics of progeny formation with F'lac in Escherichia coli K12. Plasmid. 1978 Sep;1(4):536–544. doi: 10.1016/0147-619x(78)90010-0. [DOI] [PubMed] [Google Scholar]
- Dagley S. A biochemical approach to some problems of environmental pollution. Essays Biochem. 1975;11:81–138. [PubMed] [Google Scholar]
- Dutta D., Ghosh D. K., Mishra A. K., Samanta T. B. Induction of benzo(a)pyrene hydroxylase in Aspergillus ochraceus TS: evidences of multiple forms of cytochrome P-450. Biochem Biophys Res Commun. 1983 Sep 15;115(2):692–699. doi: 10.1016/s0006-291x(83)80200-9. [DOI] [PubMed] [Google Scholar]
- Fall R. R., Brown J. L., Schaeffer T. L. Enzyme recruitment allows the biodegradation of recalcitrant branched hydrocarbons by Pseudomonas citronellolis. Appl Environ Microbiol. 1979 Oct;38(4):715–722. doi: 10.1128/aem.38.4.715-722.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feist C. F., Hegeman G. D. Phenol and benzoate metabolism by Pseudomonas putida: regulation of tangential pathways. J Bacteriol. 1969 Nov;100(2):869–877. doi: 10.1128/jb.100.2.869-877.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finette B. A., Subramanian V., Gibson D. T. Isolation and characterization of Pseudomonas putida PpF1 mutants defective in the toluene dioxygenase enzyme system. J Bacteriol. 1984 Dec;160(3):1003–1009. doi: 10.1128/jb.160.3.1003-1009.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Franklin B. L. The primary alkylsulfatase of Pseudomonas aeruginosa: inducer specificity and induction kinetics. Can J Microbiol. 1982 Dec;28(12):1296–1299. doi: 10.1139/m82-193. [DOI] [PubMed] [Google Scholar]
- GAILIUSIS J., RINNE R. W., BENEDICT C. R. PYUVATE-OXALOACETATE EXCHANGE REACTION IN BAKER'S YEAST. Biochim Biophys Acta. 1964 Dec 23;92:595–601. doi: 10.1016/0926-6569(64)90019-7. [DOI] [PubMed] [Google Scholar]
- Gaal A., Neujahr H. Y. Induction of phenol-metabolizing enzymes in Trichosporon cutaneum. Arch Microbiol. 1981 Sep;130(1):54–58. doi: 10.1007/BF00527072. [DOI] [PubMed] [Google Scholar]
- Gibson D. T. Initial reactions in the bacterial degradation of aromatic hydrocarbons. Zentralbl Bakteriol Orig B. 1976 Jul;162(1-2):157–168. [PubMed] [Google Scholar]
- Kunz D. A., Chapman P. J. Catabolism of pseudocumene and 3-ethyltoluene by Pseudomonas putida (arvilla) mt-2: evidence for new functions of the TOL (pWWO) plasmid. J Bacteriol. 1981 Apr;146(1):179–191. doi: 10.1128/jb.146.1.179-191.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law A. T., Button D. K. Modulation of affinity of a marine pseudomonad for toluene and benzene by hydrocarbon exposure. Appl Environ Microbiol. 1986 Mar;51(3):469–476. doi: 10.1128/aem.51.3.469-476.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Ariga N., Tsuda M., Deguchi K. Purification and properties of alpha-hydroxy-gamma-carboxymuconic epsilon-semialdehyde dehydrogenase. J Biochem. 1978 Apr;83(4):1125–1134. doi: 10.1093/oxfordjournals.jbchem.a132002. [DOI] [PubMed] [Google Scholar]
- Molin G., Nilsson I. Degradation of phenol by Pseudomonas putida ATCC 11172 in continuous culture at different ratios of biofilm surface to culture volume. Appl Environ Microbiol. 1985 Oct;50(4):946–950. doi: 10.1128/aem.50.4.946-950.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticello D. J., Bakker D., Finnerty W. R. Plasmid-mediated degradation of dibenzothiophene by Pseudomonas species. Appl Environ Microbiol. 1985 Apr;49(4):756–760. doi: 10.1128/aem.49.4.756-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Williams P. A. Role of catechol and the methylcatechols as inducers of aromatic metabolism in Pseudomonas putida. J Bacteriol. 1974 Mar;117(3):1153–1157. doi: 10.1128/jb.117.3.1153-1157.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick A., Weiner M. ENZYME INDUCTION AS AN ALL-OR-NONE PHENOMENON. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Rogers J. E. Kinetic concepts for measuring microbial rate constants: effects of nutrients on rate constants. Appl Environ Microbiol. 1986 Feb;51(2):221–225. doi: 10.1128/aem.51.2.221-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. R., Button D. K. Phosphate-limited continuous culture of Rhodotorula rubra: kinetics of transport, leakage, and growth. J Bacteriol. 1979 Jun;138(3):884–895. doi: 10.1128/jb.138.3.884-895.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. E., Gibson D. T. Purification and properties of cis-toluene dihydrodiol dehydrogenase from Pseudomonas putida. J Bacteriol. 1977 Jun;130(3):1117–1124. doi: 10.1128/jb.130.3.1117-1124.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariaslani F. S., Sudmeier J. L., Focht D. D. Degradation of 3-phenylbutyric acid by Pseudomonas sp. J Bacteriol. 1982 Oct;152(1):411–421. doi: 10.1128/jb.152.1.411-421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto M., Alexander M. Effect of bacterial density and substrate concentration on yield coefficients. Appl Environ Microbiol. 1985 Nov;50(5):1132–1136. doi: 10.1128/aem.50.5.1132-1136.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp R. J., Pfaender F. K. Influence of easily degradable naturally occurring carbon substrates on biodegradation of monosubstituted phenols by aquatic bacteria. Appl Environ Microbiol. 1985 Feb;49(2):394–401. doi: 10.1128/aem.49.2.394-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp R., Pfaender F. K. Influence of naturally occurring humic acids on biodegradation of monosubstituted phenols by aquatic bacteria. Appl Environ Microbiol. 1985 Feb;49(2):402–407. doi: 10.1128/aem.49.2.402-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J. B., Crawford D. L., Pometto A. L. Catabolism of substituted benzoic acids by streptomyces species. Appl Environ Microbiol. 1981 Feb;41(2):442–448. doi: 10.1128/aem.41.2.442-448.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whited G. M., McCombie W. R., Kwart L. D., Gibson D. T. Identification of cis-diols as intermediates in the oxidation of aromatic acids by a strain of Pseudomonas putida that contains a TOL plasmid. J Bacteriol. 1986 Jun;166(3):1028–1039. doi: 10.1128/jb.166.3.1028-1039.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winne D. Unstirred layer, source of biased Michaelis constant in membrane transport. Biochim Biophys Acta. 1973 Feb 27;298(1):27–31. doi: 10.1016/0005-2736(73)90005-9. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Seo C. W., Okada H. Oxidation of acyclic terpenoids by Corynebacterium sp. Appl Environ Microbiol. 1985 Apr;49(4):960–963. doi: 10.1128/aem.49.4.960-963.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eyk J., Bartels T. J. Paraffin oxidation in Pseudomonas aeruginosa. I. Induction of paraffin oxidation. J Bacteriol. 1968 Sep;96(3):706–712. doi: 10.1128/jb.96.3.706-712.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


