Abstract
Eighteen outbred mice and 21 golden hamsters were each inoculated intradermally with 2 X 10(6) Leishmania amastigotes obtained from 1 case of Sudanese cutaneous leishmaniasis. The skin lesions, spleen, lymph nodes, liver and kidney were examined by light-, polarizing-, and electron microscope at 5, 9 and 18 weeks after inoculation. The aim of the investigations was to follow the development of the inflammatory reaction and the change of the morphology of the lymphoid organs during the infection. In all the mice and in the majority of the hamsters visceral leishmaniasis developed which was characterized by a "noncure" type of cellular reaction, a selective T-cell depletion in the lymph nodes and the spleen, and the development of a reactive, systemic amyloidosis. These findings point to the failure of the acquired resistance against Leishmania to develop. In some of the hamsters the response was of the "cure" type without the development of amyloidosis. At the site of the inoculation the lesions healed suggesting the positive role of necrosis and the elimination of the parasites through the ulcer in the healing process. Electron microscopy showed erythrophagocytosis in the spleen of the 2 mice examined presenting an experimental evidence of the destruction of the red blood cells, which is a common feature of human kala-azar.
Full text
PDF

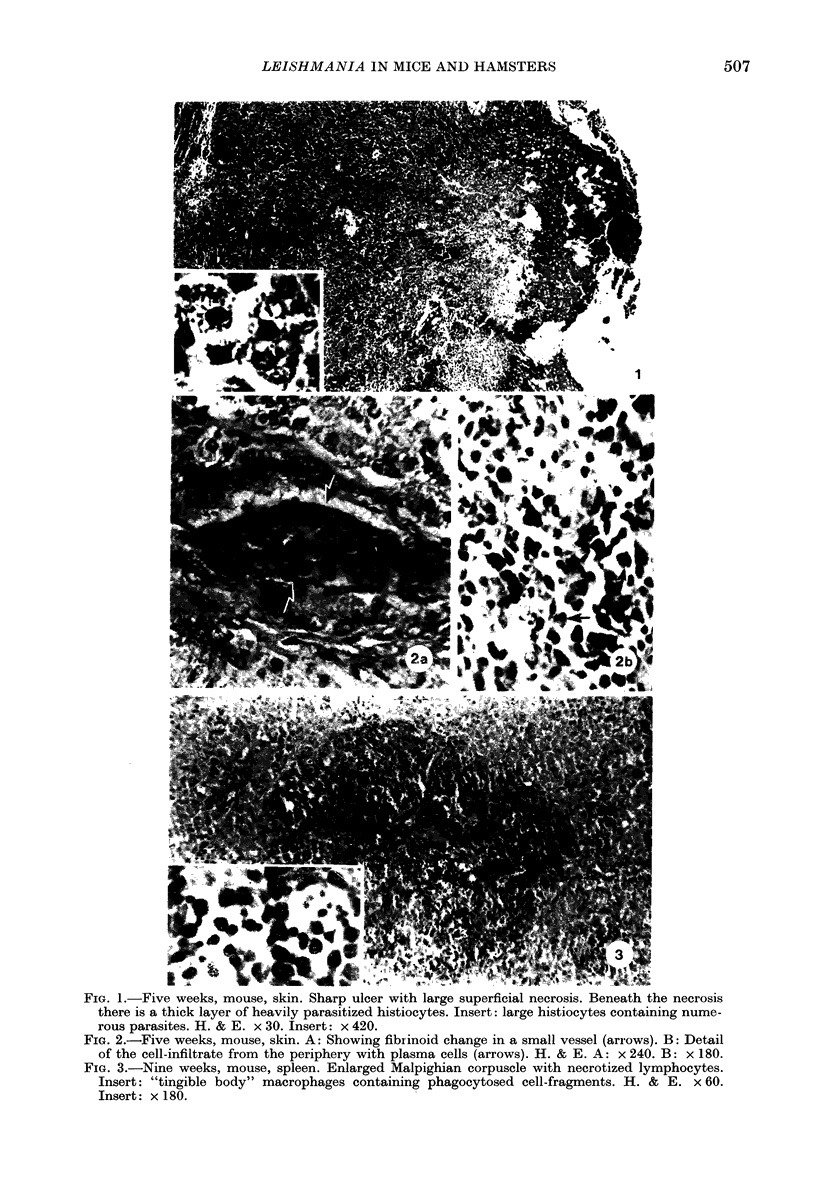

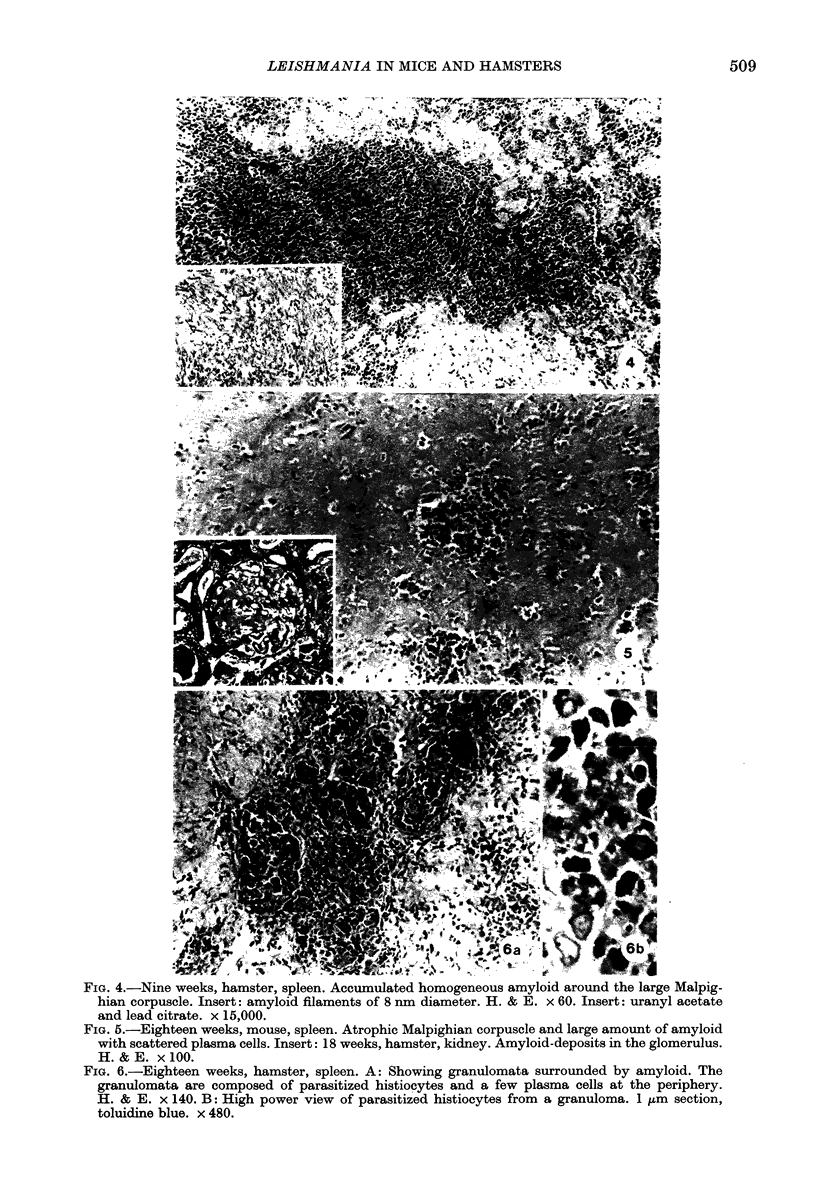




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdalla R. E., Ali M., Wasfi A. I., el-Hassan A. M. Cutaneous leishmaniasis in the Sudan. Trans R Soc Trop Med Hyg. 1973;67(4):549–559. doi: 10.1016/0035-9203(73)90086-2. [DOI] [PubMed] [Google Scholar]
- Abdalla R. E. Parasites in Sudanese cutaneous and mucosal leishmaniasis. Ann Trop Med Parasitol. 1982 Jun;76(3):299–307. doi: 10.1080/00034983.1982.11687545. [DOI] [PubMed] [Google Scholar]
- Behin R., Mauel J., Sordat B. Leishmania tropica: pathogenicity and in vitro macrophage function in strains of inbred mice. Exp Parasitol. 1979 Aug;48(1):81–91. doi: 10.1016/0014-4894(79)90057-2. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M. Genetic control of recovery from visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1982;76(2):147–151. doi: 10.1016/0035-9203(82)90262-0. [DOI] [PubMed] [Google Scholar]
- Blackwell J., Freeman J., Bradley D. Influence of H-2 complex on acquired resistance to Leishmania donovani infection in mice. Nature. 1980 Jan 3;283(5742):72–74. doi: 10.1038/283072a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Kirkley J. Regulation of Leishmania populations within the host. I. the variable course of Leishmania donovani infections in mice. Clin Exp Immunol. 1977 Oct;30(1):119–129. [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J. Letter: Genetic control of natural resistance to Leishmania donovani. Nature. 1974 Jul 26;250(464):353–354. doi: 10.1038/250353a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. J. Regulation of Leishmania populations within the host. II. genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin Exp Immunol. 1977 Oct;30(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D., Bray R. S., Wolstencroft R. A., Dumonde D. C. Immunity in cutaneous leishmaniasis of the guinea-pig. Clin Exp Immunol. 1970 Sep;7(3):301–341. [PMC free article] [PubMed] [Google Scholar]
- Cathcart E. S., Mullarkey M., Cohen A. S. Amyloidosis: an expression of immunological tolerance? Lancet. 1970 Sep 26;2(7674):639–640. doi: 10.1016/s0140-6736(70)91403-0. [DOI] [PubMed] [Google Scholar]
- Claesson M. H., Hardt F. Quantitative studies on the decay of lymphoid cells during the development of casein-induced murine amyloidosis. Acta Pathol Microbiol Scand A. 1972;80(1):125–133. doi: 10.1111/j.1699-0463.1972.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Coutinho-Abath E., Coelho M. de V. Experimental cutaneous leishmaniasis. II. The pathology of leishmaniasis by Leishmania mexicana. Rev Inst Med Trop Sao Paulo. 1965 May-Jun;7(3):145–155. [PubMed] [Google Scholar]
- DeTolla L. J., Scott P. A., Farrell J. P. Single gene control of resistance to cutaneous leishmaniasis in mice. Immunogenetics. 1981;14(1-2):29–39. doi: 10.1007/BF00344297. [DOI] [PubMed] [Google Scholar]
- Decker-Jackson J. E., Honigberg B. M. Glycoproteins released by Leishmania donovani: immunologic relationships with host and bacterial antigens and preliminary biochemical analysis. J Protozool. 1978 Nov;25(4):514–525. doi: 10.1111/j.1550-7408.1978.tb04178.x. [DOI] [PubMed] [Google Scholar]
- Djoko-Tamnou J., Leclerc C., Modabber F., Chedid L. Studies on visceral Leishmania tropica infection in BALB/c mice. I. Clinical features and cellular changes. Clin Exp Immunol. 1981 Dec;46(3):493–498. [PMC free article] [PubMed] [Google Scholar]
- Duarte M. I., Sesso A., de Brito T. Relationship between glomerular mesangial cell proliferation and amyloid deposition as seen by ultrastructural and morphometric analysis in experimental kala-azar of the hamster. Am J Pathol. 1978 Jul;92(1):85–98. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Infectious immunological tolerance. Immunology. 1971 Dec;21(6):903–914. [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Handman E., Ceredig R., Mitchell G. F. Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust J Exp Biol Med Sci. 1979 Feb;57(1):9–29. doi: 10.1038/icb.1979.2. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Chan-Liew W. L. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 1980 Winter;2(4):303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Genetically determined response mechanisms to cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1982;76(2):152–154. doi: 10.1016/0035-9203(82)90263-2. [DOI] [PubMed] [Google Scholar]
- Leclerc C., Modabber F., Deriaud E., Cheddid L. Systemic infection of Leishmania tropica (major) in various strains of mice. Trans R Soc Trop Med Hyg. 1981;75(6):851–854. doi: 10.1016/0035-9203(81)90430-2. [DOI] [PubMed] [Google Scholar]
- Leclerc C., Modabber F., Deriaud E., Djoko-Tamnou J., Chedid L. Visceral Leishmania tropica infection of BALB/c mice: cellular analysis of in vitro unresponsiveness to sheep erythrocytes. Infect Immun. 1982 Sep;37(3):895–902. doi: 10.1128/iai.37.3.895-902.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosev B., Daoud E. H., El Hadi A., El Hassan A. M., Sati M. H. Mucosal leishmaniasis in the Sudan. Ann Trop Med Parasitol. 1969 Mar;63(1):123–128. doi: 10.1080/00034983.1969.11686607. [DOI] [PubMed] [Google Scholar]
- Monroy A., Ridley D. S., Heather C. J., Ridley M. J. Histological studies of the elimination of Leishmania enriettii from skin lesions in the guinea-pig. Br J Exp Pathol. 1980 Dec;61(6):601–610. [PMC free article] [PubMed] [Google Scholar]
- Nasseri M., Modabber F. Z. Generalized infection and lack of delayed hypersensitivity in BALB/c mice infected with Leishmania tropica major. Infect Immun. 1979 Nov;26(2):611–614. doi: 10.1128/iai.26.2.611-614.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez H., Labrador F., Torrealba J. W. Variations in the response of five strains of mice to Leishmania mexicana. Int J Parasitol. 1979 Feb;9(1):27–32. doi: 10.1016/0020-7519(79)90062-6. [DOI] [PubMed] [Google Scholar]
- Rezai H. R., Farrell J., Soulsby E. L. Immunological responses of L. donovani infection in mice and significance of T cell in resistance to experimental leishmaniasis. Clin Exp Immunol. 1980 Jun;40(3):508–514. [PMC free article] [PubMed] [Google Scholar]
- Ridley D. S. The pathogenesis of cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1979;73(2):150–160. doi: 10.1016/0035-9203(79)90199-8. [DOI] [PubMed] [Google Scholar]
- Veress B., Abdalla R. E., El Hassan A. M. Electron microscope investigations on leishmaniasis in the Sudan. I. Morphometric studies on Leishmania amastigotes in various forms of human leishmaniasis. Ann Trop Med Parasitol. 1980 Aug;74(4):421–426. doi: 10.1080/00034983.1980.11687363. [DOI] [PubMed] [Google Scholar]
- Woodruff A. W. Mechanisms involved in anaemia associated with infection and splenomegaly in the tropics. Trans R Soc Trop Med Hyg. 1973;67(3):313–328. doi: 10.1016/0035-9203(73)90107-7. [DOI] [PubMed] [Google Scholar]