Abstract
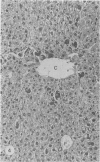
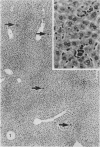
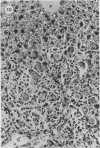
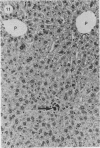
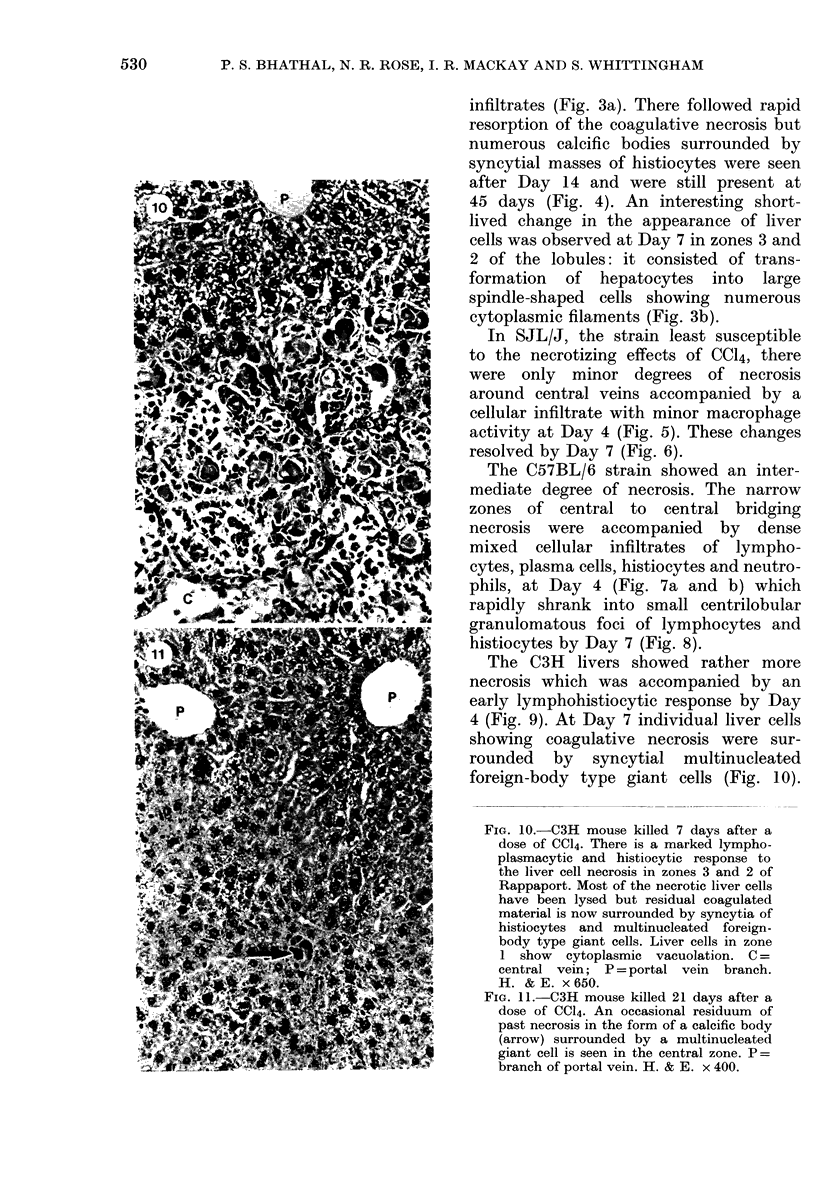
A study was made in inbred mice of genetic determinants of toxic liver cell injury, and the subsequent occurrence of autoantibodies to mouse liver-specific protein (M-LSP). Carbon tetrachloride was injected s.c. in sublethal doses to induce liver cell damage in 4 strains of mice, BALB/c, C3H, C57BL/6 and SJL/J. The degree of liver cell damage was assessed by blood cholylglycine levels and by semiquantitative histological analysis 1, 4, 7, 14, 21 and 45 days after dosing. Striking differences were observed among the 4 strains in degrees of liver necrosis, cellular infiltration and rate of removal of the necrotic tissue. BALB/c was the strain most susceptible to the necrotizing effects of CCl4. These mice showed confluent areas of hepatocellular necrosis from Day 1 and histological recovery was protracted up to 3 weeks, and was accompanied by pronounced macrophage activity and a cellular inflammatory response. Mice of the SJL/J strain were the least susceptible and showed minor hepatocellular necrosis, which resolved by Day 7, and a slight histiocytic response. C3H and C57BL/6 showed lesions which were intermediate between the other 2 strains. The autoantibody response to LSP was weak, transient and detected only in C57BL/6 mice. This study indicates the presence of genetic control, either H-2 or non-H-2 linked, over the degree of liver cell necrosis resulting from toxic liver injury, and the ensuing cellular infiltrate and rate of removal of the necrotic tissue.
Full text
PDF

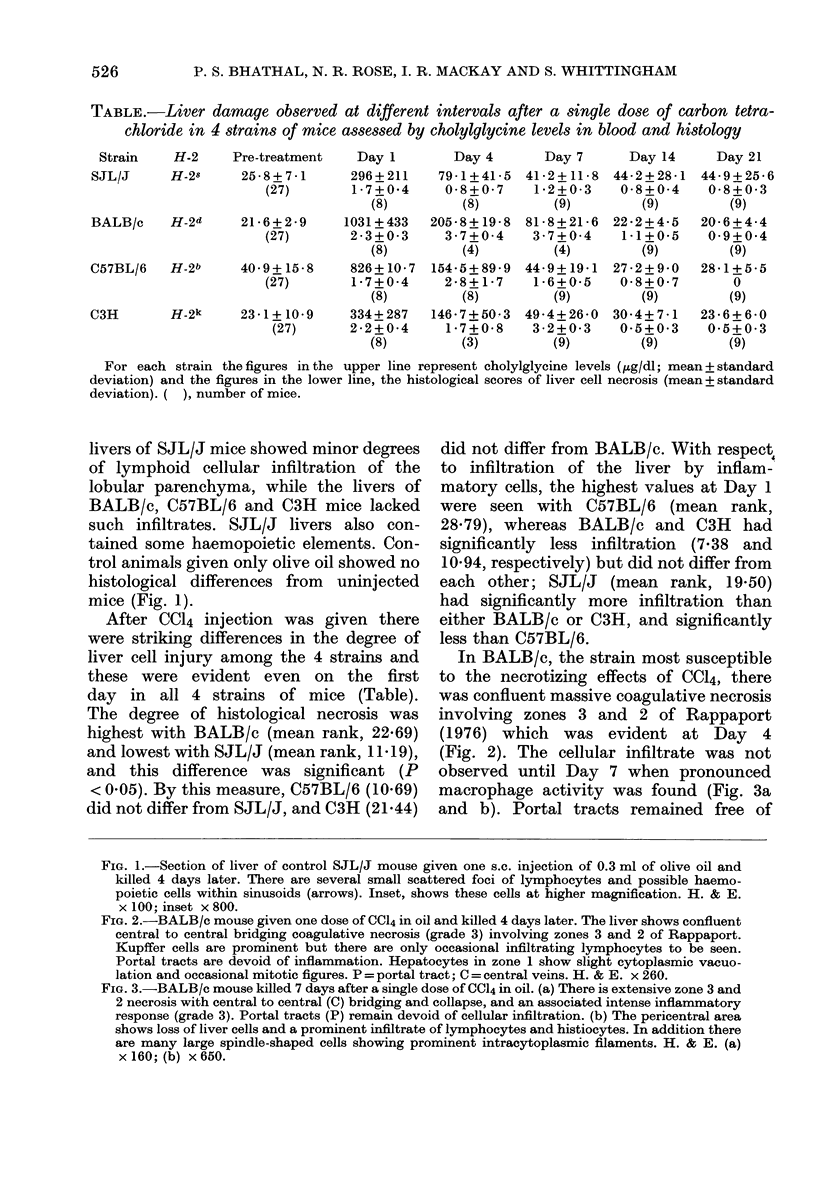
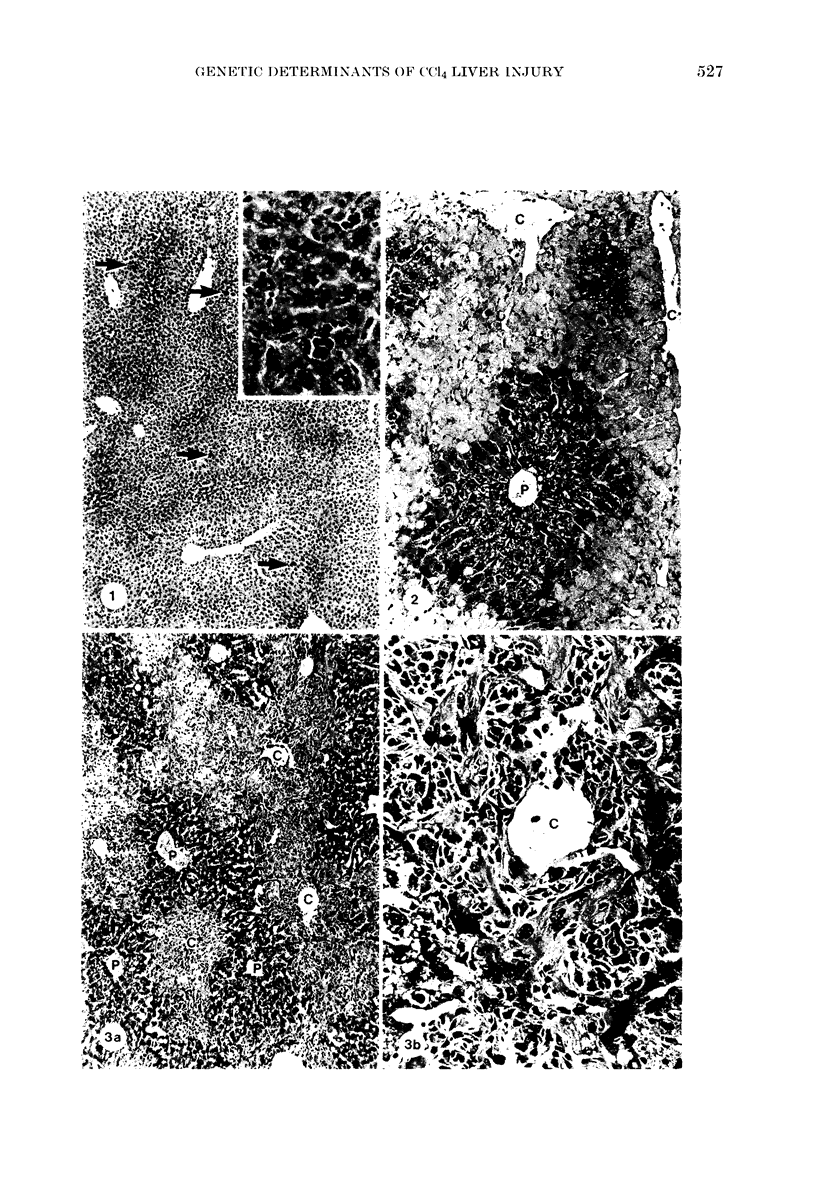
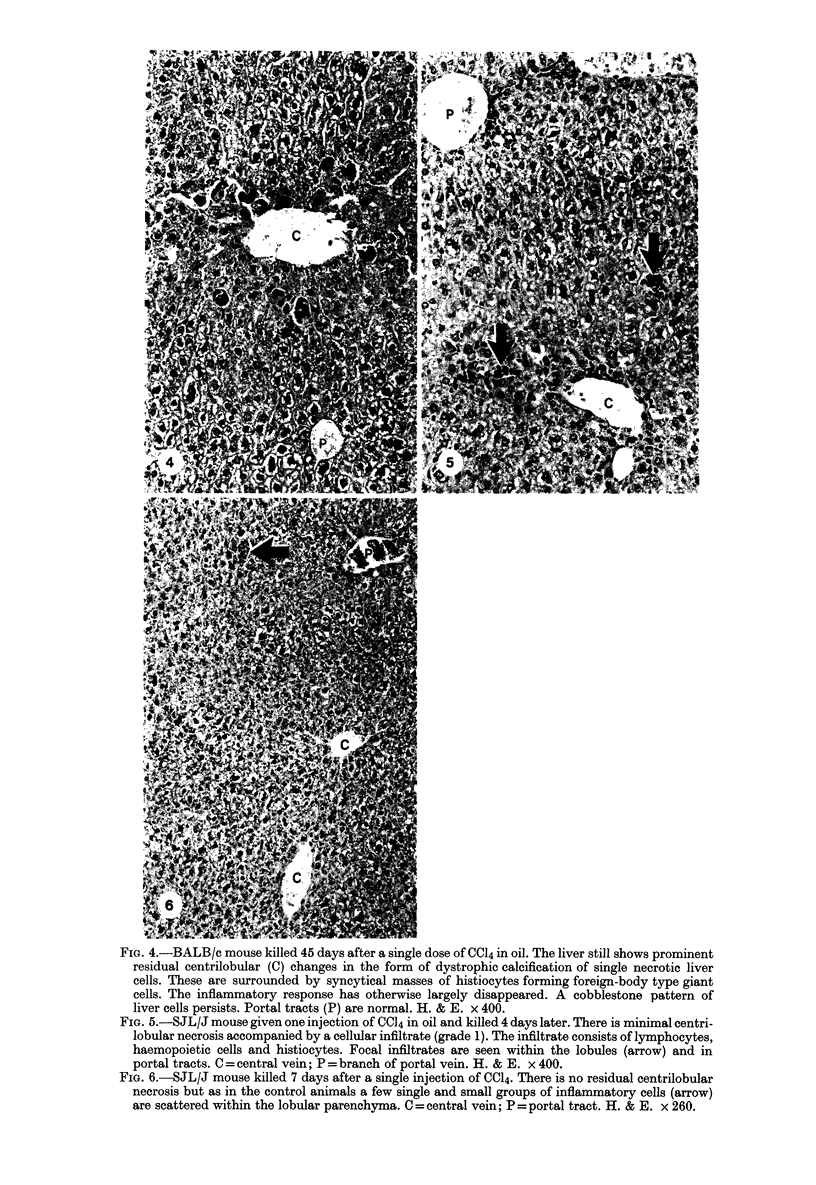
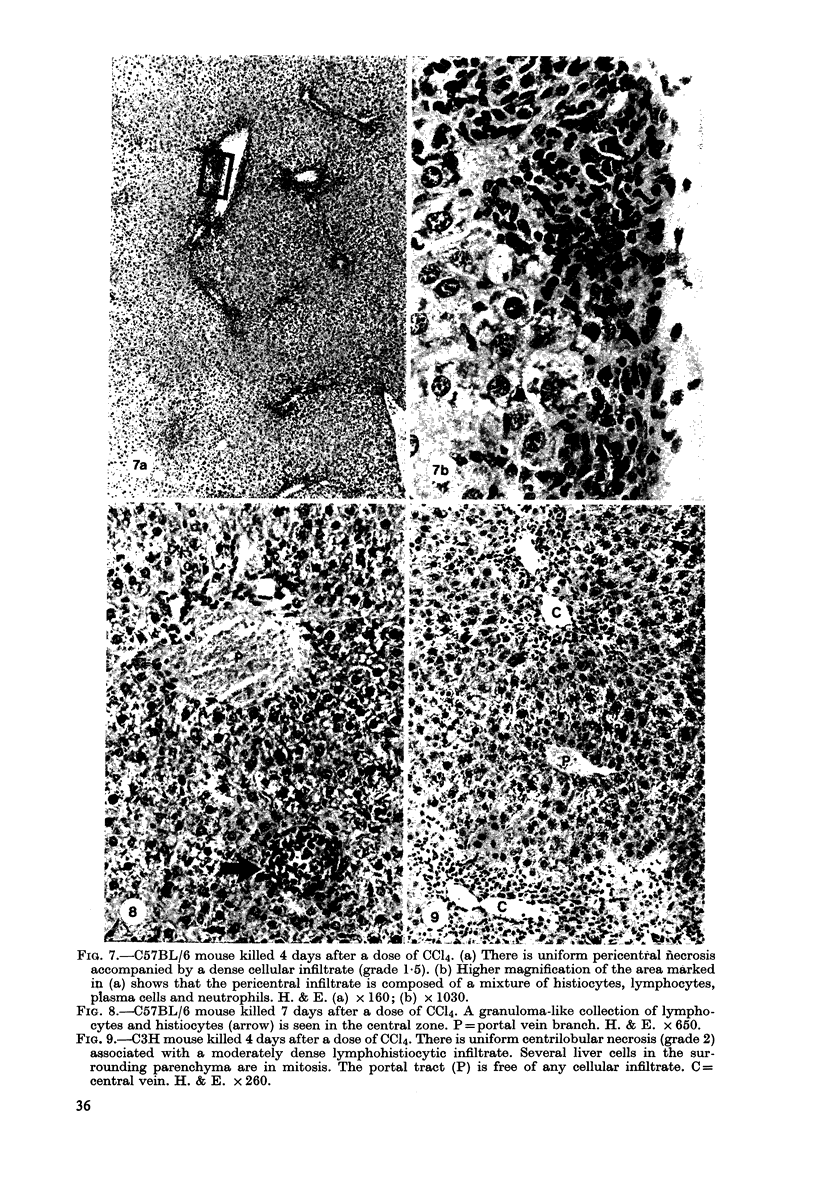



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Jensen D. M., McFarlane I. G., Portmann B. S., Eddleston A. L., Williams R. Detection of antibodies directed against a liver-specific membrane lipoprotein in patients with acute and chronic active hepatitis. N Engl J Med. 1978 Jul 6;299(1):1–7. doi: 10.1056/NEJM197807062990101. [DOI] [PubMed] [Google Scholar]
- Meyer zum Büschenfelde K. H., Manns M., Hütteroth T. H., Hopf U., Arnold W. LM-Ag and LSP--two different target antigens involved in the immunopathogenesis of chronic active hepatitis? Clin Exp Immunol. 1979 Aug;37(2):205–212. [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W. Genetic differences in susceptibility to chemically induced myelotoxicity and leukemia. Environ Health Perspect. 1981 Jun;39:11–22. doi: 10.1289/ehp.813911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport A. M. The microcirculatory acinar concept of normal and pathological hepatic structure. Beitr Pathol. 1976 May;157(3):215–243. doi: 10.1016/s0005-8165(76)80083-2. [DOI] [PubMed] [Google Scholar]
- Recknagel R. O. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967 Jun;19(2):145–208. [PubMed] [Google Scholar]
- Shichi H., Nebert D. W. Genetic differences in drug metabolism associated with ocular toxicity. Environ Health Perspect. 1982 Apr;44:107–117. doi: 10.1289/ehp.8244107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffelaar J. W., Ten Kate F. J., Nap M., Swaak A. J., De Graaffreitsma C. B., Van Elven E. H., Feltkamp-Vroom T. M. Immune complex detection by immunofluorescence on polymorphonuclear leucocytes. Clin Exp Immunol. 1977 Mar;27(3):391–396. [PMC free article] [PubMed] [Google Scholar]
- Weir D. M., Pinckard R. N. Failure to induce tolerance to rat tissue antigens. Immunology. 1967 Oct;13(4):373–380. [PMC free article] [PubMed] [Google Scholar]