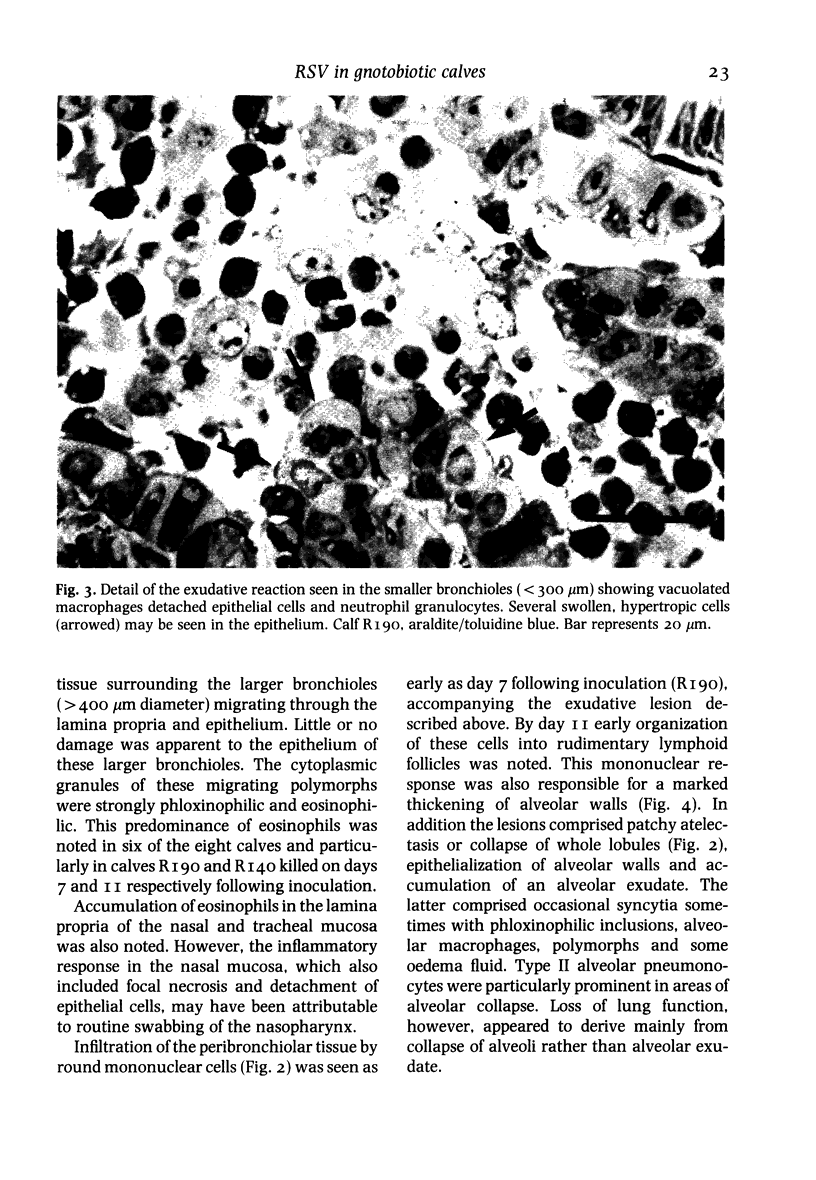
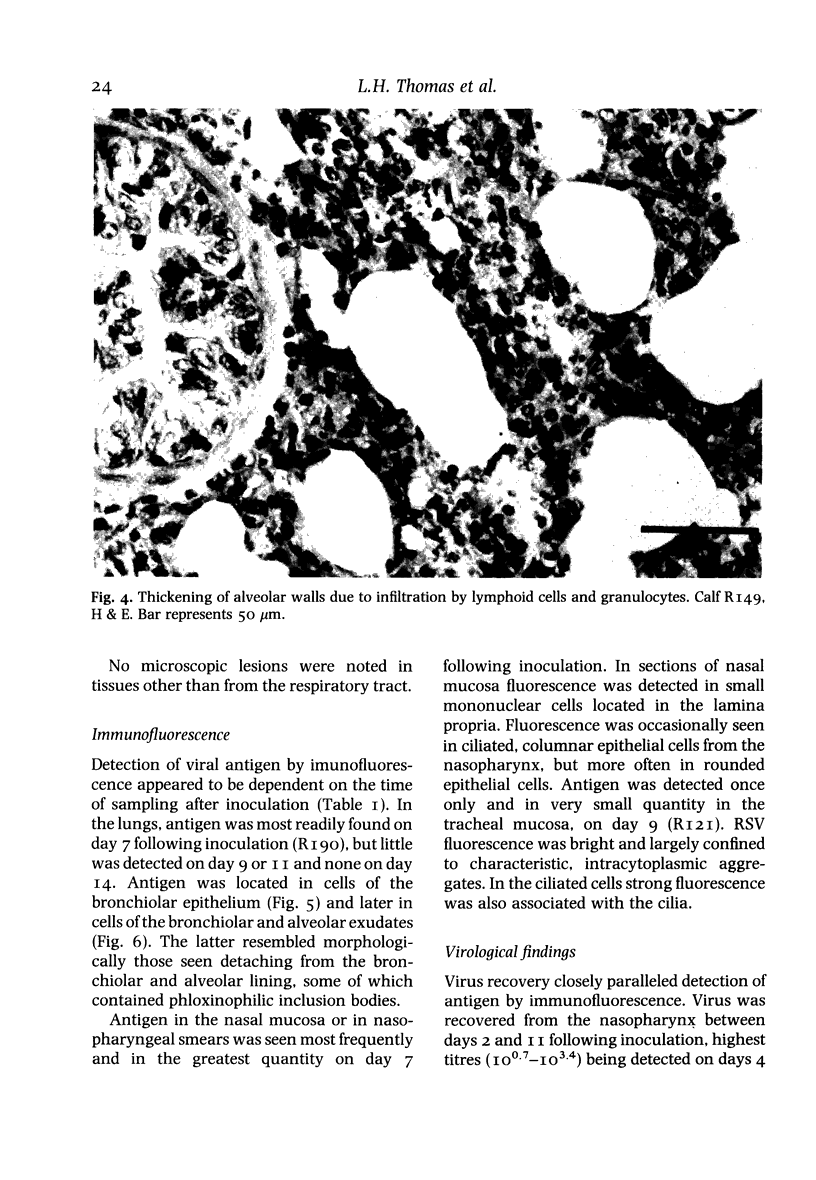
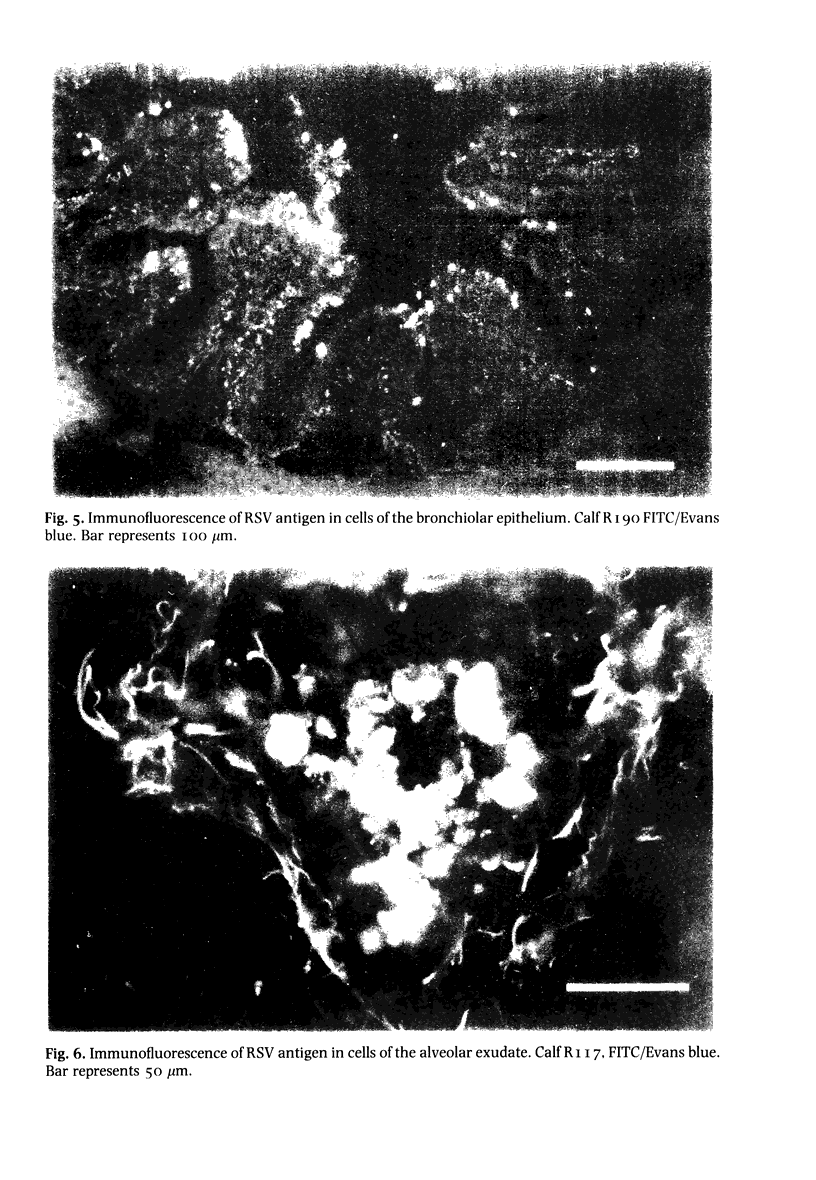
Abstract
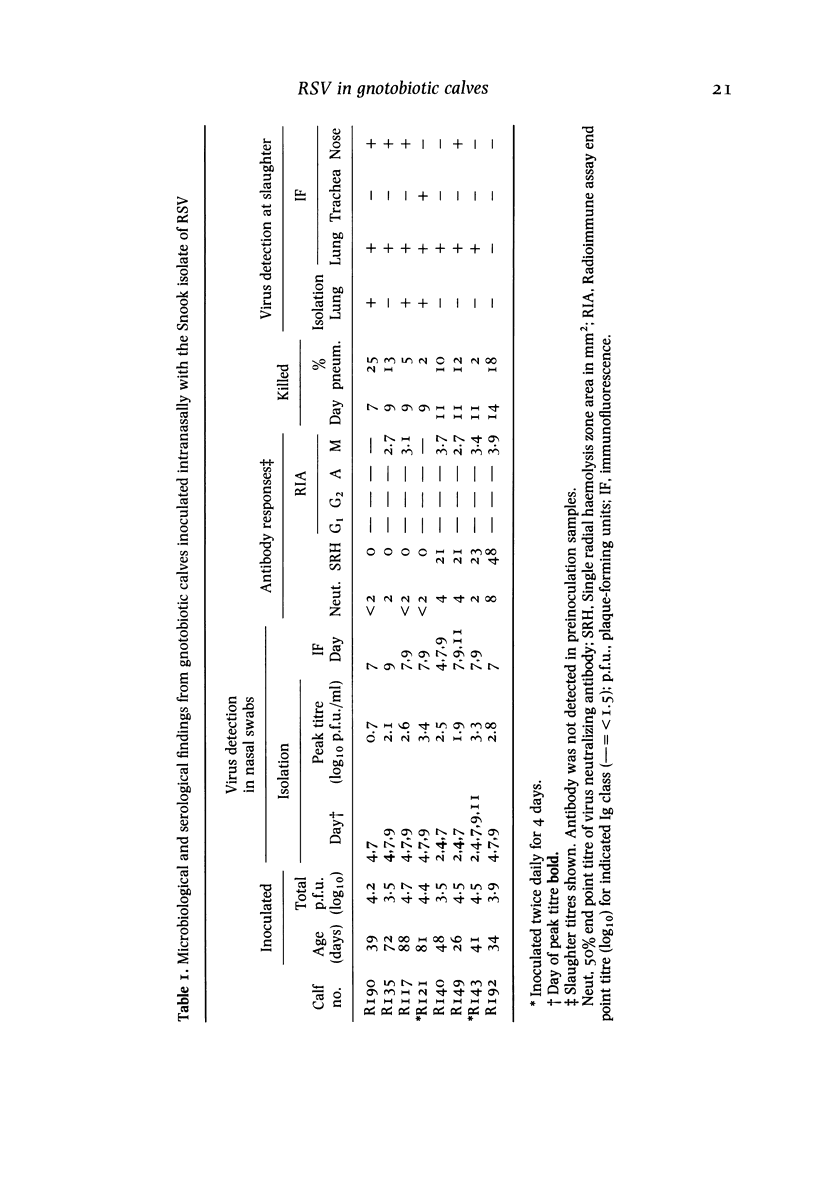
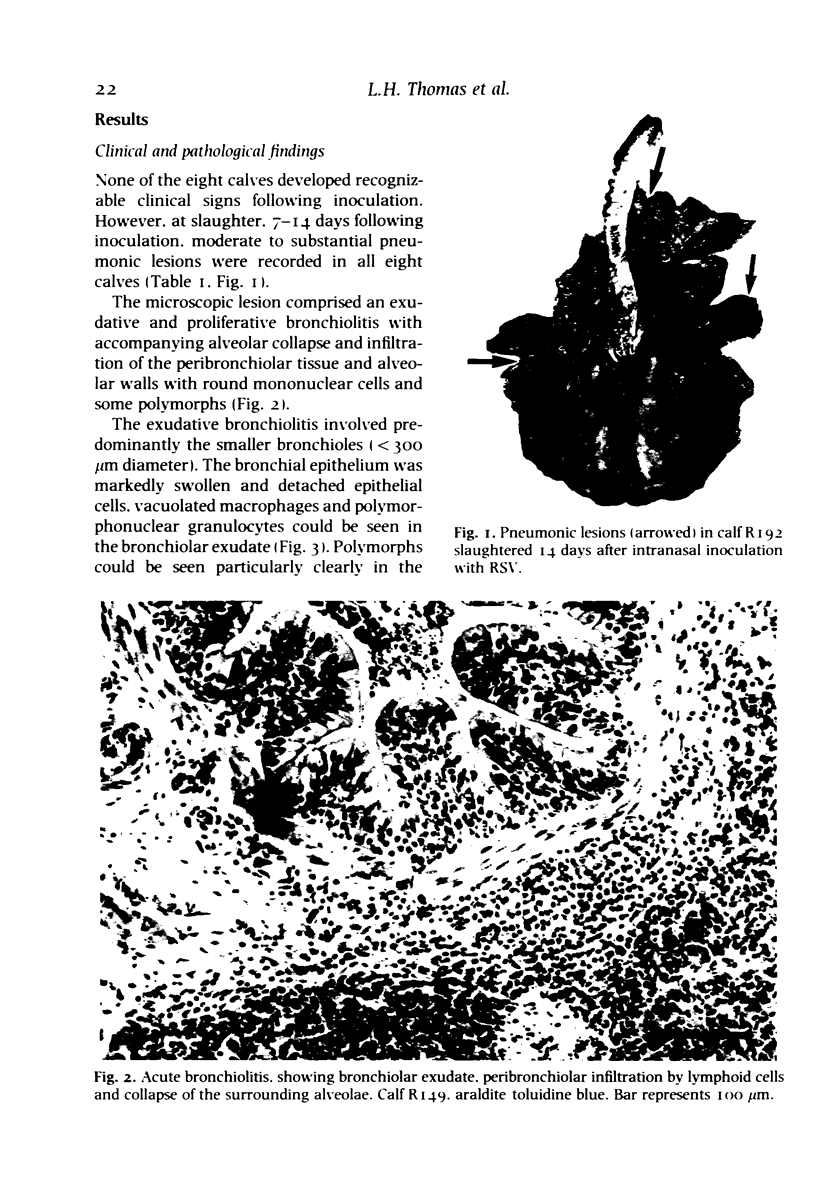
A bovine isolate of respiratory syncytial virus (RSV), when inoculated intranasally into eight gnotobiotic calves produced significant macroscopic lesions of the lung (2-25% consolidation) but failed to produce any clinical signs of disease. The microscopic lesions comprised proliferative and exudative bronchiolitis with accompanying alveolar collapse and infiltration by mononuclear cells of the peribronchiolar tissue and alveolar walls. Virus was recovered from the nasopharynx between days 2 and 11 after infection with peak titres between days 4 and 7. Demonstration of viral antigen by immunofluorescence in nasopharyngeal cells followed a similar detection pattern. Virus was recovered from lung or detected by immunofluorescence in the bronchiolar epithelium up to 11 days following inoculation. A serological response to RSV was demonstrated both by virus neutralization and single radial haemolysis (SRH) tests, in serum of calves from 11 days following inoculation. Specific anti-RSV IgM was detected from 9 days following infection. It is suggested that the close resemblance between the experimental disease in calves and the pathology of acute bronchiolitis in children make cattle a particularly relevant model for the human disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aherne W., Bird T., Court S. D., Gardner P. S., McQuillin J. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970 Feb;23(1):7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsch V., Friis N. F., Krogh H. V. A microbiological study of pneumonic calf lungs. Acta Vet Scand. 1976;17(1):32–42. doi: 10.1186/BF03547941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson D. G., McNulty M. S., Logan E. F., Cush P. F. Respiratory syncytial virus pneumonia in young calves: clinical and pathologic findings. Am J Vet Res. 1983 Sep;44(9):1648–1655. [PubMed] [Google Scholar]
- Hauser K. J., Zabransky R. J. Modification of the gas-liquid chromatography procedure and evaluation of a new column packing material for the identification of anaerobic bacteria. J Clin Microbiol. 1975 Jul;2(1):1–7. doi: 10.1128/jcm.2.1.1-7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzhauer C., Van Nieuwstadt A. P. De Etiologische Rol Van Het Bovine Respiratory Syncytial Virus Bij Pinkengriep. Voorlopige mededeling. Tijdschr Diergeneeskd. 1976 Sep 15;101(18):1023–1031. [PubMed] [Google Scholar]
- Inaba Y., Tanaka Y., Sato K., Ito H., Omori T. Nomi virus, a virus isolated from an apparently new epizootic respiratory disease of cattle. Jpn J Microbiol. 1970 May;14(3):246–248. doi: 10.1111/j.1348-0421.1970.tb00518.x. [DOI] [PubMed] [Google Scholar]
- Jacobs J. W., Edington N. Experimental infection of calves with respiratory syncytial virus. Res Vet Sci. 1975 May;18(3):299–306. [PubMed] [Google Scholar]
- McNulty M. S., Bryson D. G., Allan G. M. Experimental respiratory syncytial virus pneumonia in young calves: microbiologic and immunofluorescent findings. Am J Vet Res. 1983 Sep;44(9):1656–1659. [PubMed] [Google Scholar]
- Mengeling W. L. Porcine parvovirus: frequency of naturally occurring transplacental infection and viral contamination of fetal porcine kidney cell cultures. Am J Vet Res. 1975 Jan;36(1):41–44. [PubMed] [Google Scholar]
- Nuttall P. A., Luther P. D., Stott E. J. Viral contamination of bovine foetal serum and cell cultures. Nature. 1977 Apr 28;266(5605):835–837. doi: 10.1038/266835a0. [DOI] [PubMed] [Google Scholar]
- Paccaud M. F., Jacquier C. A respiratory syncytial virus of bovine origin. Arch Gesamte Virusforsch. 1970;30(4):327–342. doi: 10.1007/BF01258363. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Jenson A. B., Horswood R. L., Camargo E., Chanock R. M. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978 Dec;93(3):771–791. [PMC free article] [PubMed] [Google Scholar]
- Rosenquist B. D. Isolation of respiratory syncytial virus from calves with acute respiratory disease. J Infect Dis. 1974 Aug;130(2):177–182. doi: 10.1093/infdis/130.2.177. [DOI] [PubMed] [Google Scholar]
- Taylor G. Solid-phase micro-radioimmunoassay to measure immunoglobulin class-specific antibody to Mycoplasma pulmonis. Infect Immun. 1979 Jun;24(3):701–706. doi: 10.1128/iai.24.3.701-706.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L. H., Gourlay R. N., Stott E. J., Howard C. J., Bridger J. C. A search for new microorganisms in calf pneumonia by the inoculation of gnotobiotic calves. Res Vet Sci. 1982 Sep;33(2):170–182. doi: 10.1016/S0034-5288(18)32331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Ingh T. S., Verhoeff J., Van Nieuwstadt A. P. Clinical and pathological observations on spontaneous bovine respiratory syncytial virus infections in calves. Res Vet Sci. 1982 Sep;33(2):152–158. [PubMed] [Google Scholar]