Abstract
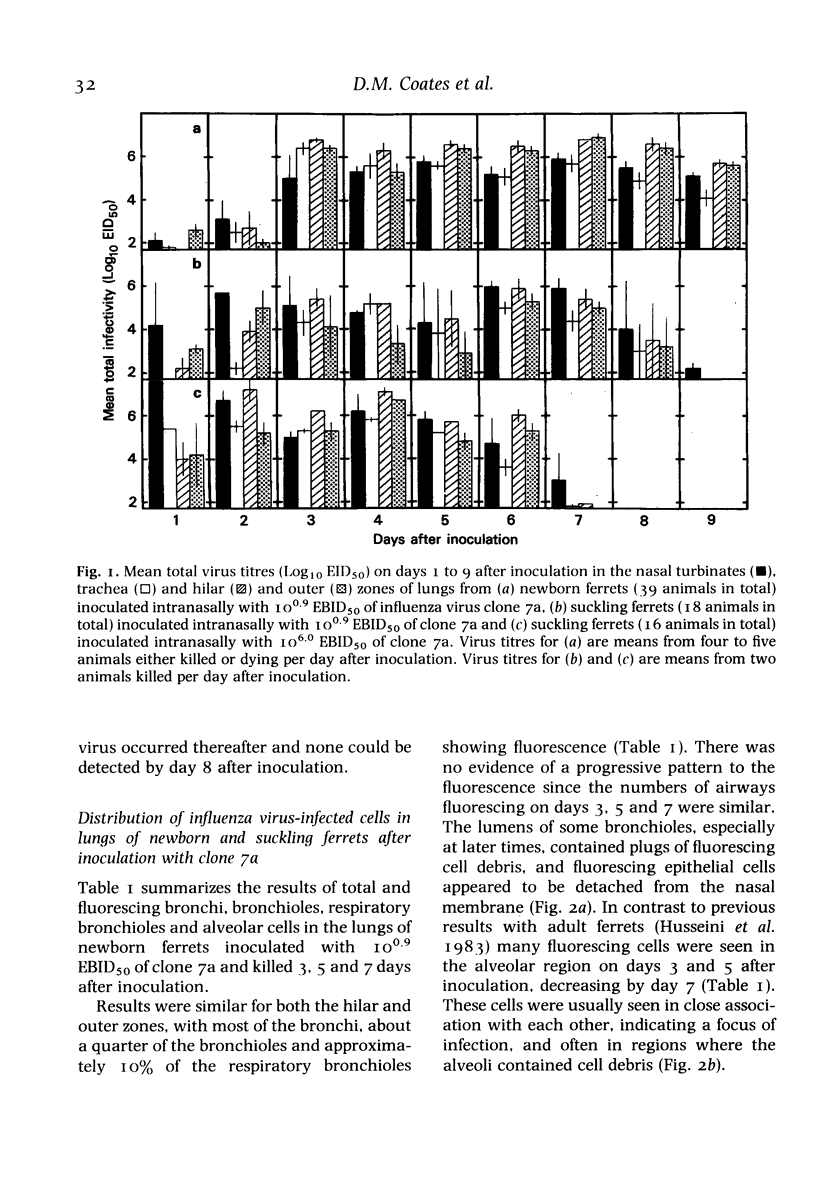
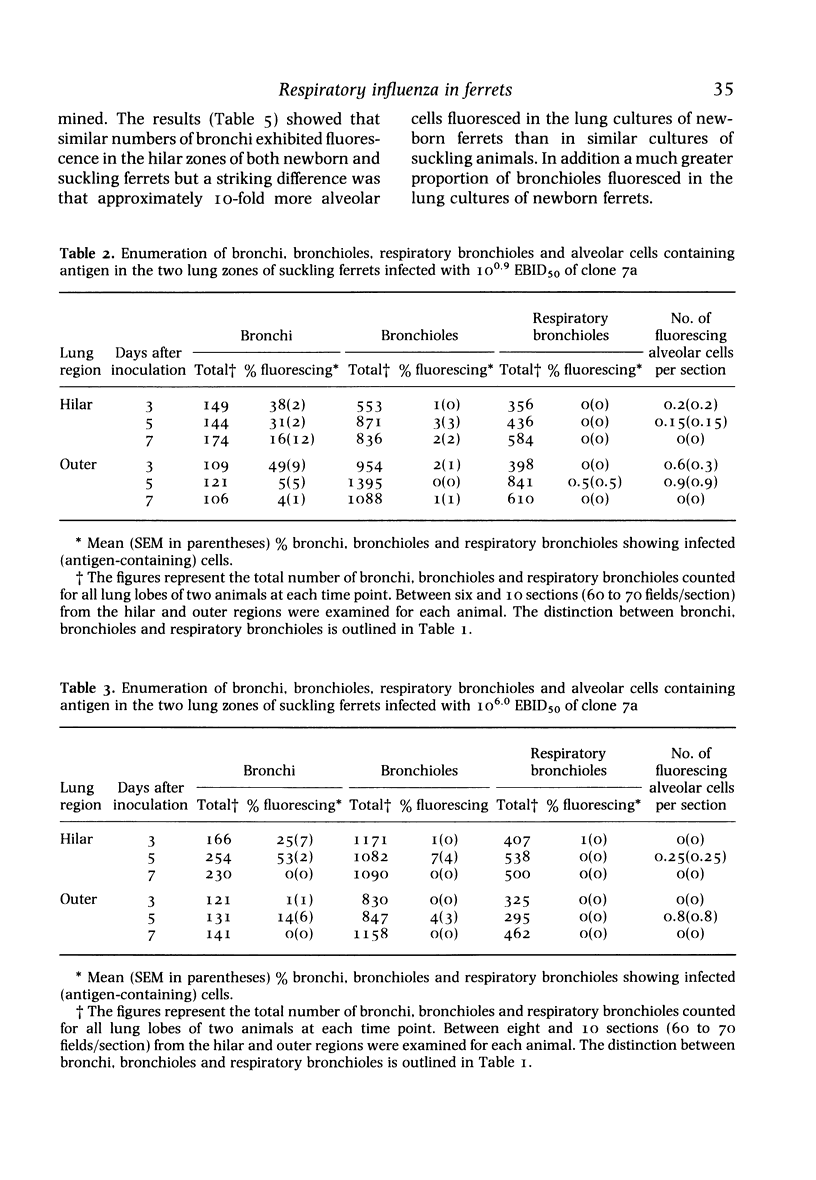
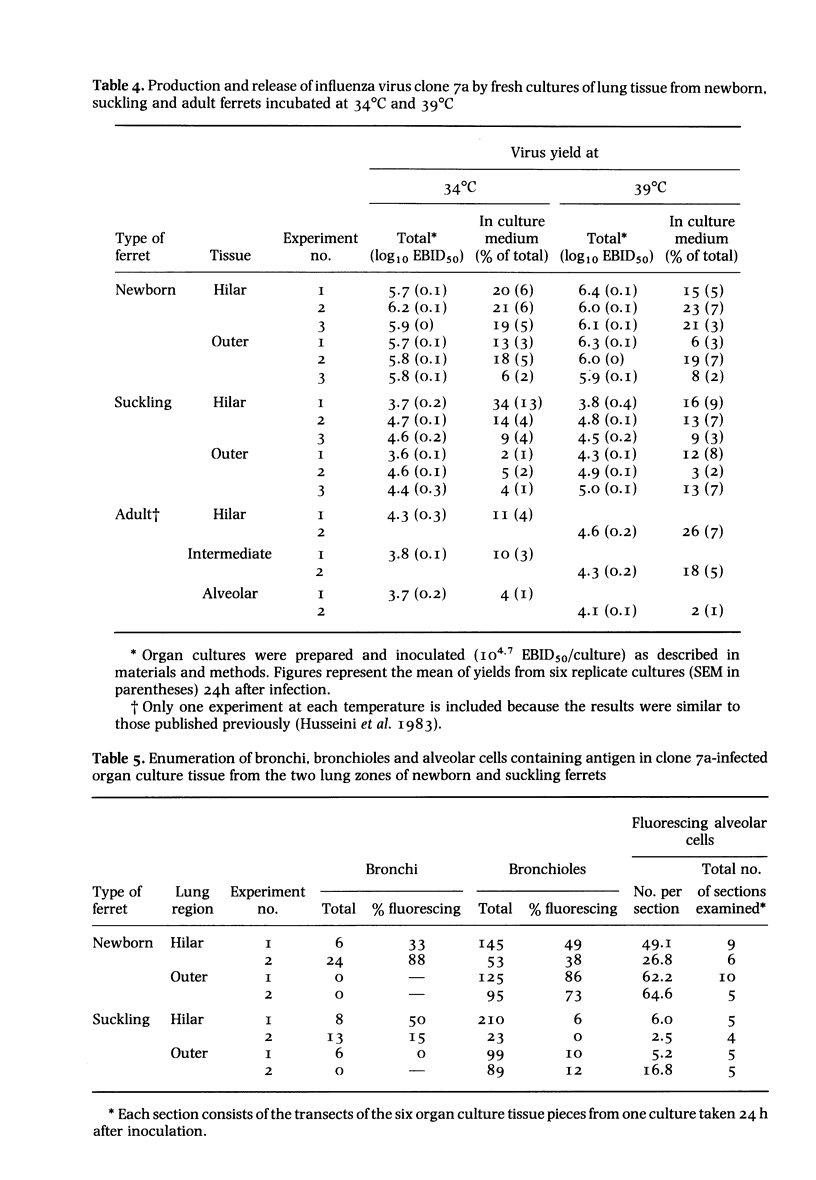
A comparison was made, both in vivo and in organ culture, between newborn (1-day-old) and suckling (15-day-old) ferrets of lower respiratory tract tissue infected with a virulent strain (clone 7a) of influenza virus. Newborn ferrets were killed by influenza virus following intranasal inoculation but suckling ferrets were almost as resistant as adult ferrets. In newborn ferrets there was a rapid, severe and progressive infection of lung tissue with infection of alveolar cells as well as those of bronchial and bronchiolar epithelium (assessed by monitoring virus infectivity and by fluorescent antibody staining). In suckling ferrets, as previously shown for adult animals, the lung infection was less severe, less persistent and confined to the epithelium of bronchi with only a small bronchiolar involvement and even less alveolar cell infection. These differences observed in vivo were repeated in organ cultures obtained from various areas of the lung. i.e. alveolar and airway epithelial cells of newborn ferrets exhibited a greater susceptibility than those of older ferrets. Thus, it appears that one factor determining the greater susceptibility of the lower respiratory tract of newborn ferrets is a greater inherent susceptibility of alveolar and airway epithelial cells to infection with influenza virus. Other factors may also be involved and have yet to be investigated.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Skoglund A. C., Rönnholm M., Lindsten T., Lamon E. W., Collisson E. W., Walia A. S. Functional aspects of IgM and IgG Fc receptors on murine T lymphocytes. Immunol Rev. 1981;56:5–50. doi: 10.1111/j.1600-065x.1981.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Belshe R. B., Richardson L. S., Prevar D. A., Camargo E., Chanock R. M. Growth and genetic stability of 4 temperature sensitive (ts) mutants of respiratory syncytial (RS) virus in newborn ferrets. Arch Virol. 1978;58(4):313–321. doi: 10.1007/BF01317823. [DOI] [PubMed] [Google Scholar]
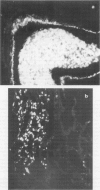
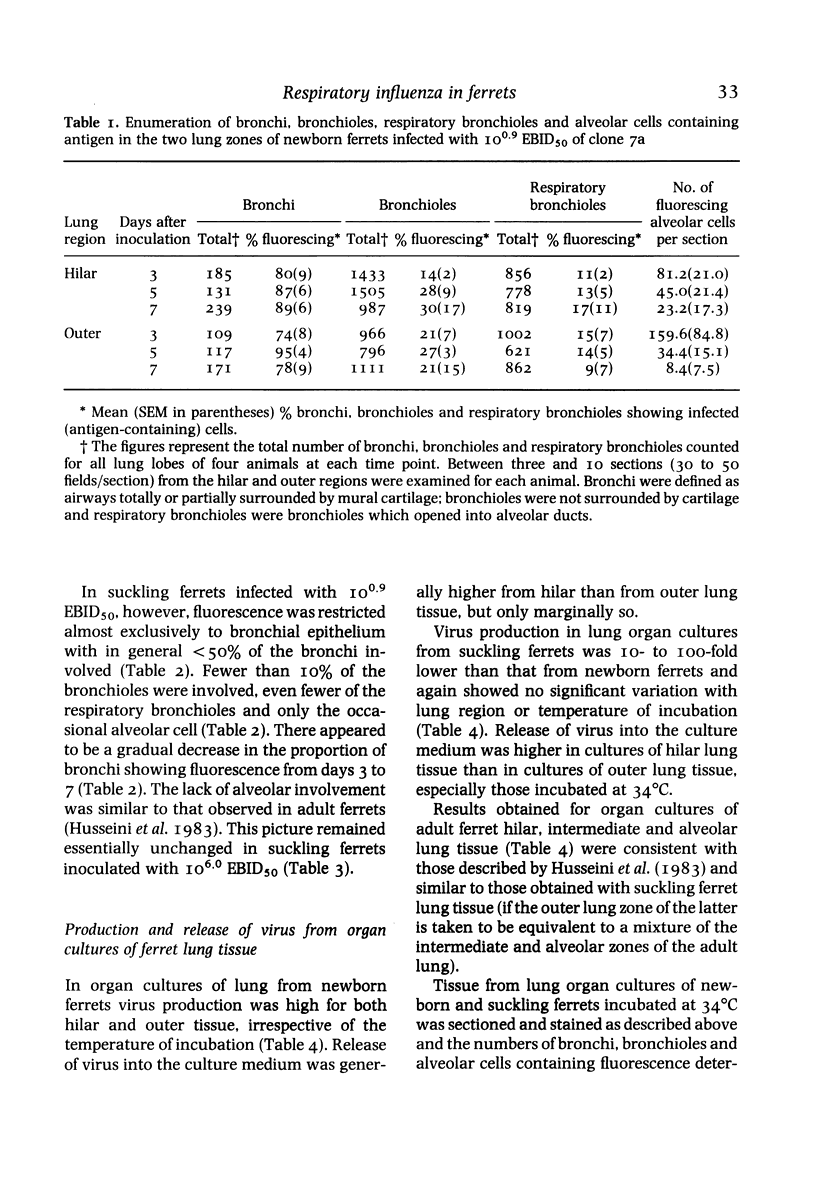
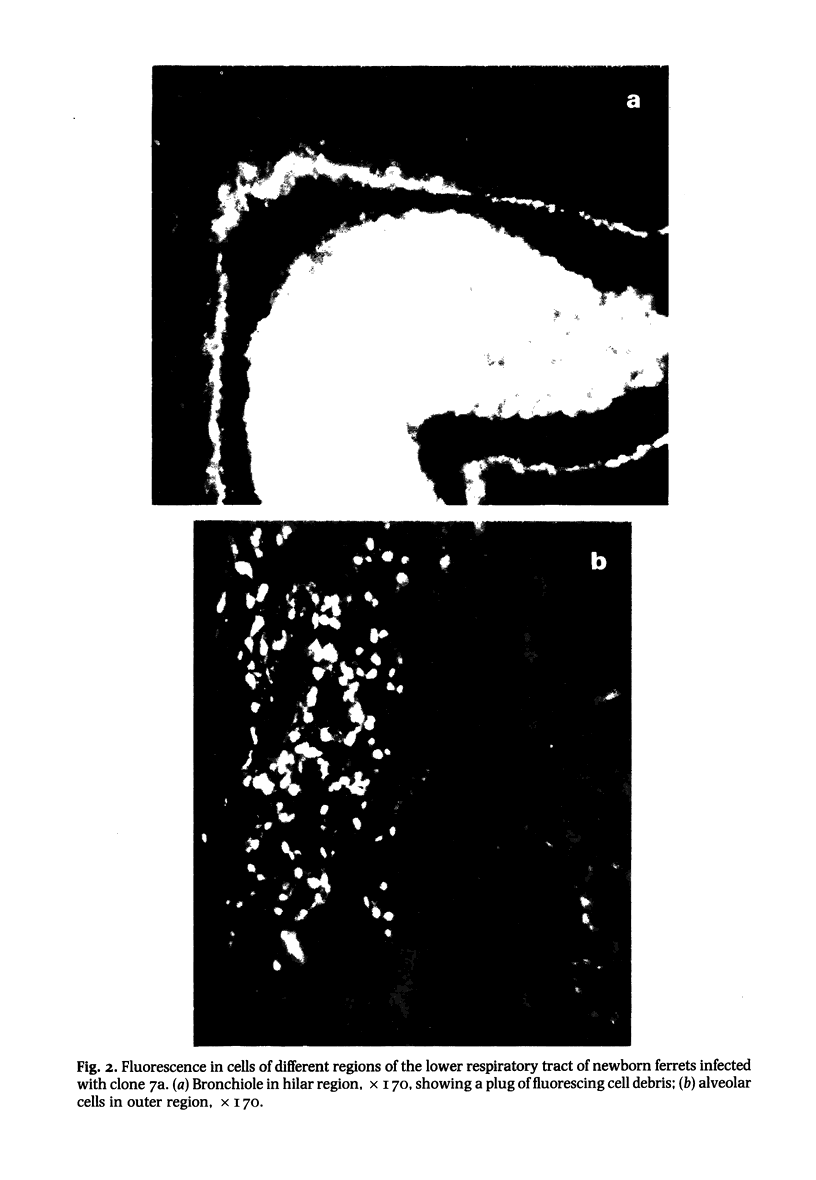
- Husseini R. H., Sweet C., Bird R. A., Collie M. H., Smith H. Distribution of viral antigen with the lower respiratory tract of ferrets infected with a virulent influenza virus: production and release of virus from corresponding organ cultures. J Gen Virol. 1983 Mar;64(Pt 3):589–598. doi: 10.1099/0022-1317-64-3-589. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Brandt C. D., Arrobio J. O., Murphy B., Chanock R. M., Parrott R. H. Influenza A and B virus infection in infants and young children during the years 1957-1976. Am J Epidemiol. 1979 Apr;109(4):464–479. doi: 10.1093/oxfordjournals.aje.a112704. [DOI] [PubMed] [Google Scholar]
- Kingsman S., Toms G. L., Smith H. The localization of influenza virus in the respiratory tract of ferrets: the different susceptibilities of fresh and maintained organ cultures. J Gen Virol. 1977 Nov;37(2):249–258. doi: 10.1099/0022-1317-37-2-249. [DOI] [PubMed] [Google Scholar]
- Laraya-Cuasay L. R., DeForest A., Huff D., Lischner H., Huang N. N. Chronic pulmonary complications of early influenza virus infection in children. Am Rev Respir Dis. 1977 Oct;116(4):617–625. doi: 10.1164/arrd.1977.116.4.617. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979 Mar;43(1):1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisley J. W., Bruhn F. W., Lauer B. A., McIntosh K. Type A2 influenza viral infections in children. Am J Dis Child. 1978 Jan;132(1):34–36. doi: 10.1001/archpedi.1978.02120260036007. [DOI] [PubMed] [Google Scholar]
- Pittard W. B., 3rd Breast milk immunology. A frontier in infant nutrition. Am J Dis Child. 1979 Jan;133(1):83–87. doi: 10.1001/archpedi.1979.02130010089019. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Muck K. B., Prince G. A. The age dependence of respiratory syncytial virus growth in ferret lung can be shown in organ and monolayer cultures. Clin Immunol Immunopathol. 1980 Mar;15(3):415–423. doi: 10.1016/0090-1229(80)90053-7. [DOI] [PubMed] [Google Scholar]
- Poulsen S. S., Szabo S. Mucosal surface morphology and histological changes in the duodenum of the rat following administration of cysteamine. Br J Exp Pathol. 1977 Feb;58(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Porter D. D. The pathogenesis of respiratory syncytial virus infection in infant ferrets. Am J Pathol. 1976 Feb;82(2):339–352. [PMC free article] [PubMed] [Google Scholar]
- Roberts N. J., Jr Temperature and host defense. Microbiol Rev. 1979 Jun;43(2):241–259. doi: 10.1128/mr.43.2.241-259.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet C., Macartney J. C., Bird R. A., Cavanagh D., Collie M. H., Husseini R. H., Smith H. Differential distribution of virus and histological damage in the lower respiratory tract of ferrets infected with influenza viruses of differing virulence. J Gen Virol. 1981 May;54(Pt 1):103–114. doi: 10.1099/0022-1317-54-1-103. [DOI] [PubMed] [Google Scholar]
- Welsh R. M. Natural cell-mediated immunity during viral infections. Curr Top Microbiol Immunol. 1981;92:83–106. doi: 10.1007/978-3-642-68069-4_6. [DOI] [PubMed] [Google Scholar]