Abstract
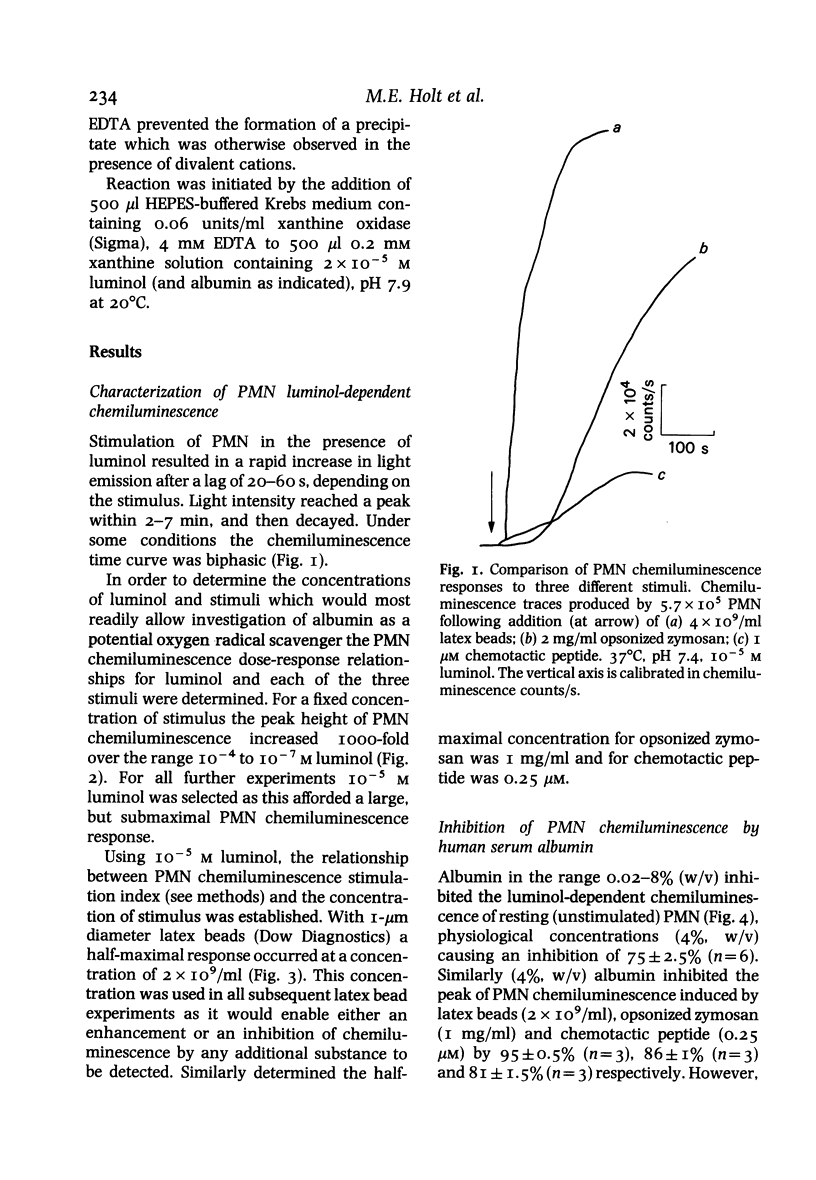
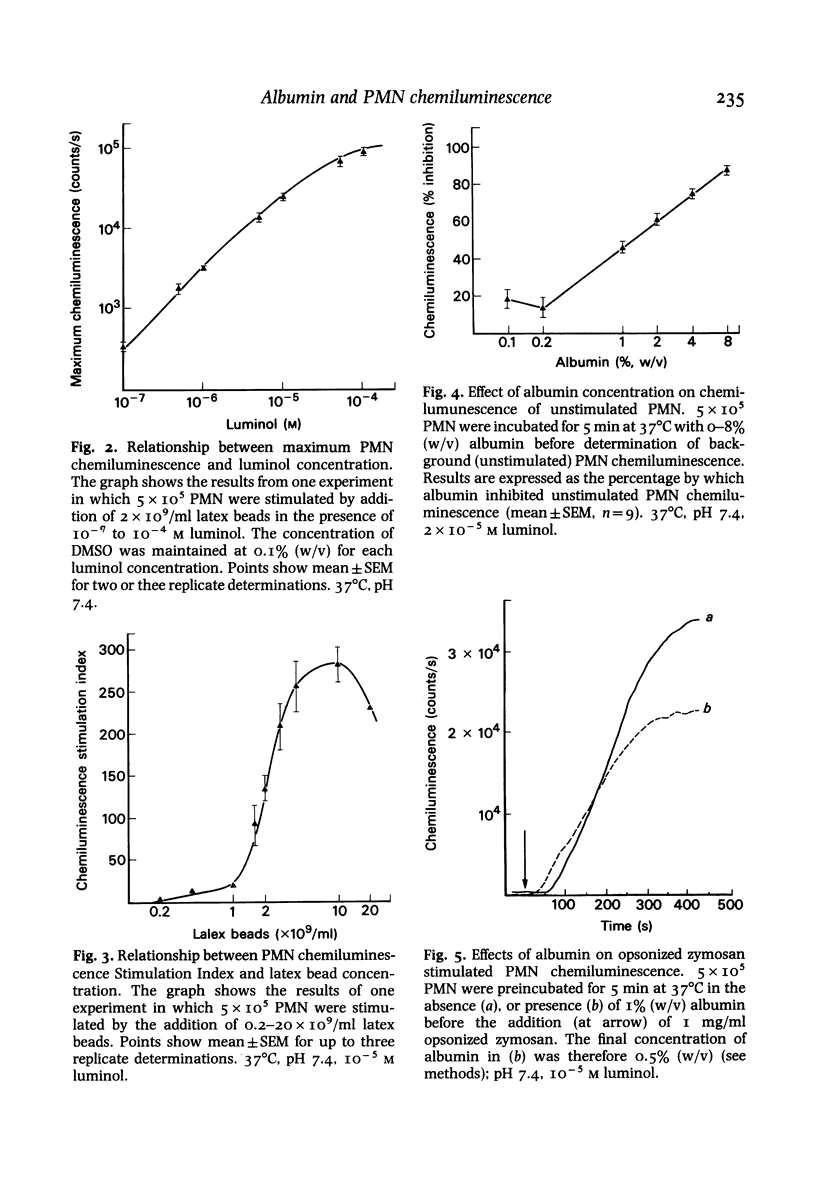
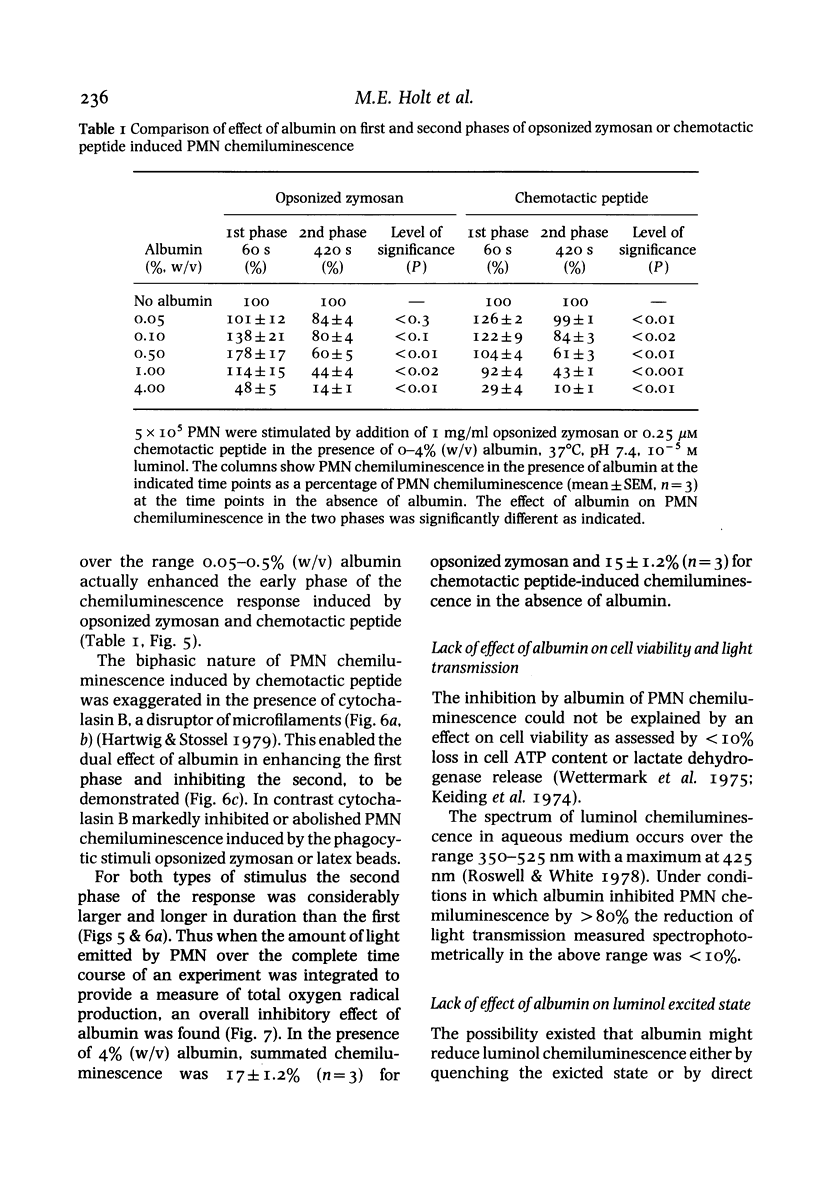
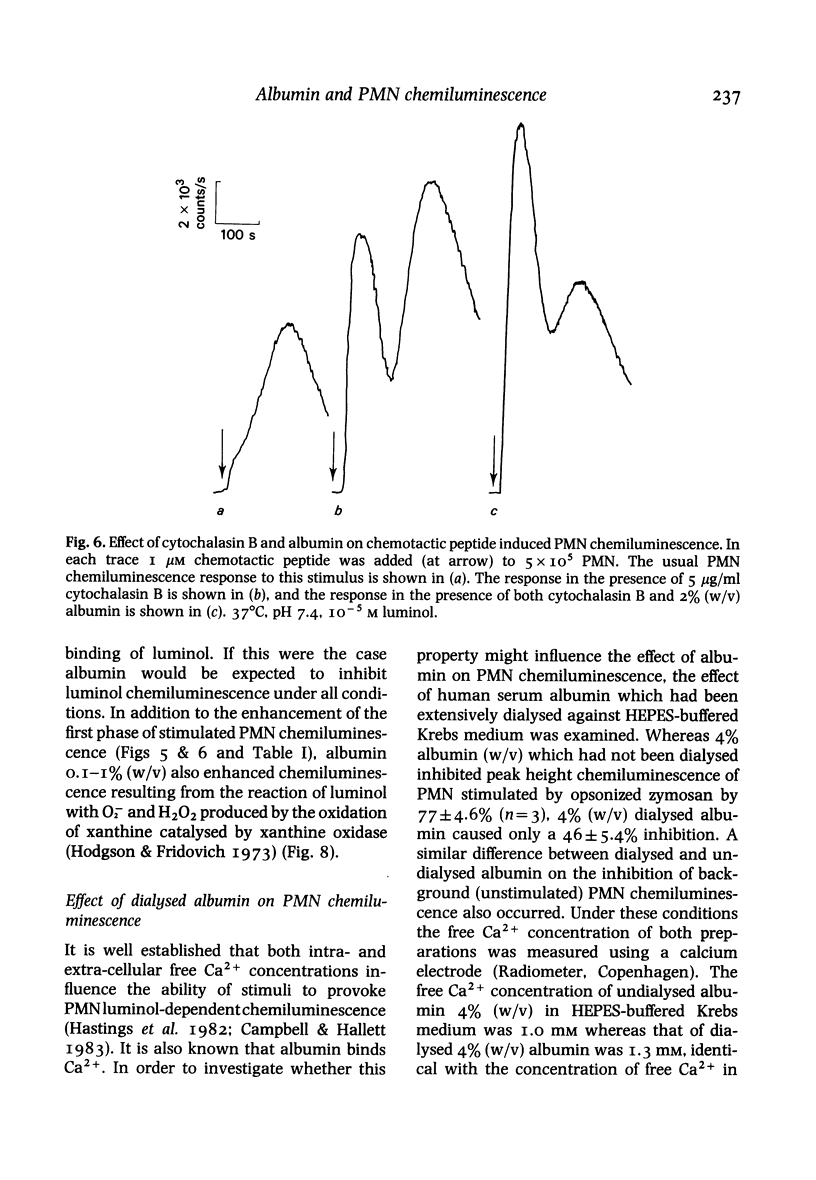
Luminol-dependent chemiluminescence of normal human polymorphonuclear leucocytes (PMN) which were resting, or stimulated by unopsonized latex beads, opsonized zymosan or the chemotactic peptide N-formyl-met-leu-phe was decreased more than 80% in the presence of physiological concentrations of albumin (4%, w/v). This inhibition did not result from impairment of light transmission, cellular toxicity, luminol excited-state quenching or a dialysable contaminant in the albumin preparation, but was reduced by 30% when the fall induced by albumin in extracellular free Ca2+ concentration was corrected. The inhibition was most apparent in the larger second phase of the PMN chemiluminescent response to chemotactic peptide or opsonized zymosan stimulation. The smaller first phase of these responses was in fact enhanced by low concentrations of albumin (0.05-0.5%, w/v) and only inhibited up to 50% by 4% (w/v) albumin. Albumin in the range 0.1-4% (w/v) exerted a similar effect on chemiluminescence resulting from superoxide anion (O-2) and hydrogen peroxide (H2O2) production by xanthine oxidase catalysed oxidation of xanthine in the presence of luminol. We suggest that the effect of albumin on PMN luminol-dependent chemiluminescence is mediated by modification of the oxygen radical generating pathway, or oxygen radical scavenging. This previously undocumented property of the major extracellular protein requires further examination if oxygen radicals are to be established as important mediators of inflammation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell A. K., Hallett M. B. Measurement of intracellular calcium ions and oxygen radicals in polymorphonuclear leucocyte-erythrocyte 'ghost' hybrids. J Physiol. 1983 May;338:537–550. doi: 10.1113/jphysiol.1983.sp014688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Maestro R. F. An approach to free radicals in medicine and biology. Acta Physiol Scand Suppl. 1980;492:153–168. [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easmon C. S., Cole P. J., Williams A. J., Hastings M. The measurement of opsonic and phagocytic function by Luminol-dependent chemiluminescence. Immunology. 1980 Sep;41(1):67–74. [PMC free article] [PubMed] [Google Scholar]
- Evans P. H. Serum sulphydryl levels in rheumatoid patients treated with alclofenac. Curr Med Res Opin. 1975;3(5):268–275. doi: 10.1185/03007997509114777. [DOI] [PubMed] [Google Scholar]
- Hastings M. J., Petricevic I., Williams A. J., Cole P. J., Easmon C. S. The effect of culture media on the production and measurement of luminol-dependent chemiluminescence. Br J Exp Pathol. 1982 Apr;63(2):147–153. [PMC free article] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The role of O2- in the chemiluminescence of luminol. Photochem Photobiol. 1973 Dec;18(6):451–455. doi: 10.1111/j.1751-1097.1973.tb06449.x. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Herron M. J., Schmidtke J. R., Simmons R. L. Chemiluminescence response of human leukocytes: influence of medium components on light production. Infect Immun. 1977 Sep;17(3):513–520. doi: 10.1128/iai.17.3.513-520.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recommended methods for the determination of four enzymes in blood. Scand J Clin Lab Invest. 1974 Jun;33(4):291–306. doi: 10.1080/00365517409082499. [DOI] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOOG W. A., BECK W. S. Studies on the fibrinogen, dextran and phytohemagglutinin methods of isolating leukocytes. Blood. 1956 May;11(5):436–454. [PubMed] [Google Scholar]
- Williams A. J., Cole P. J. Polymorphonuclear leucocyte membrane-stimulated oxidative metabolic activity---the effect of divalent cations and cytochalasins. Immunology. 1981 Dec;44(4):847–858. [PMC free article] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Domański J., Ostrowski W. Chloramines as intermediates of oxidation reaction of amino acids by myeloperoxidase. Biochim Biophys Acta. 1971 Jun 16;235(3):419–424. doi: 10.1016/0005-2744(71)90281-6. [DOI] [PubMed] [Google Scholar]


