Abstract
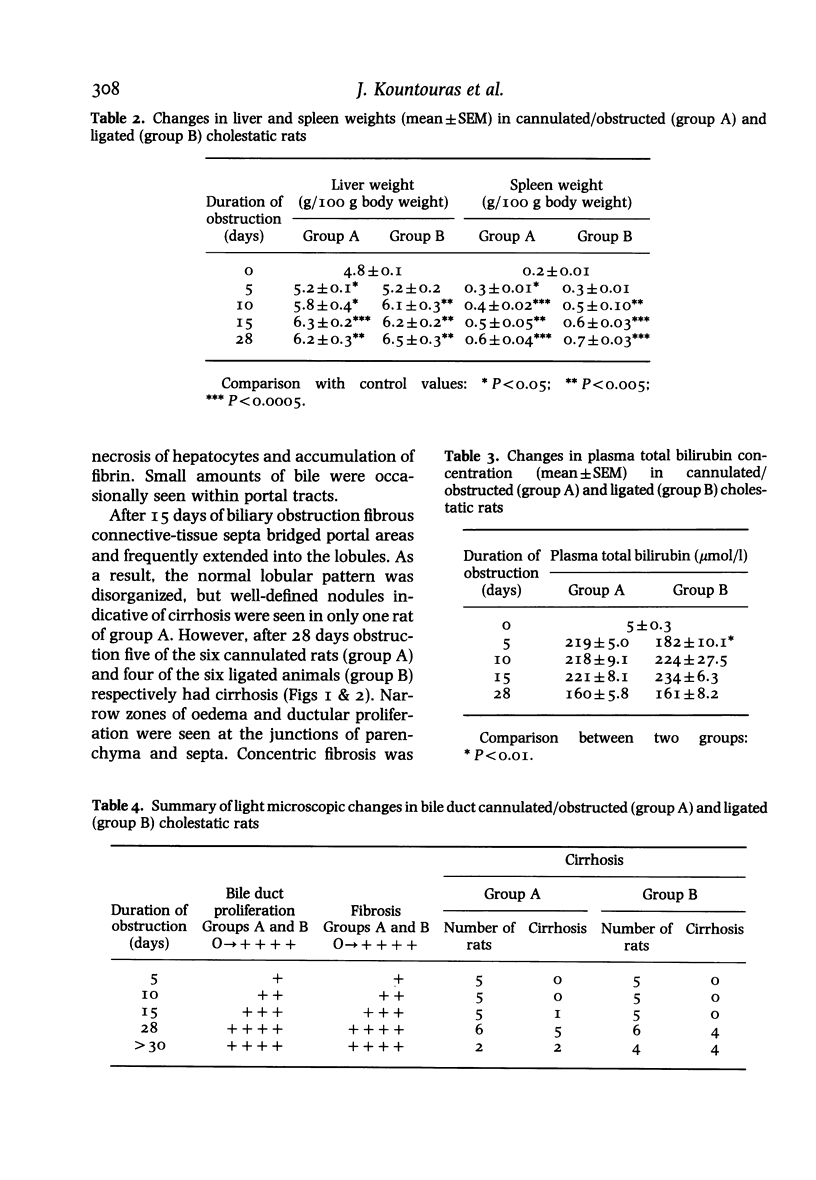
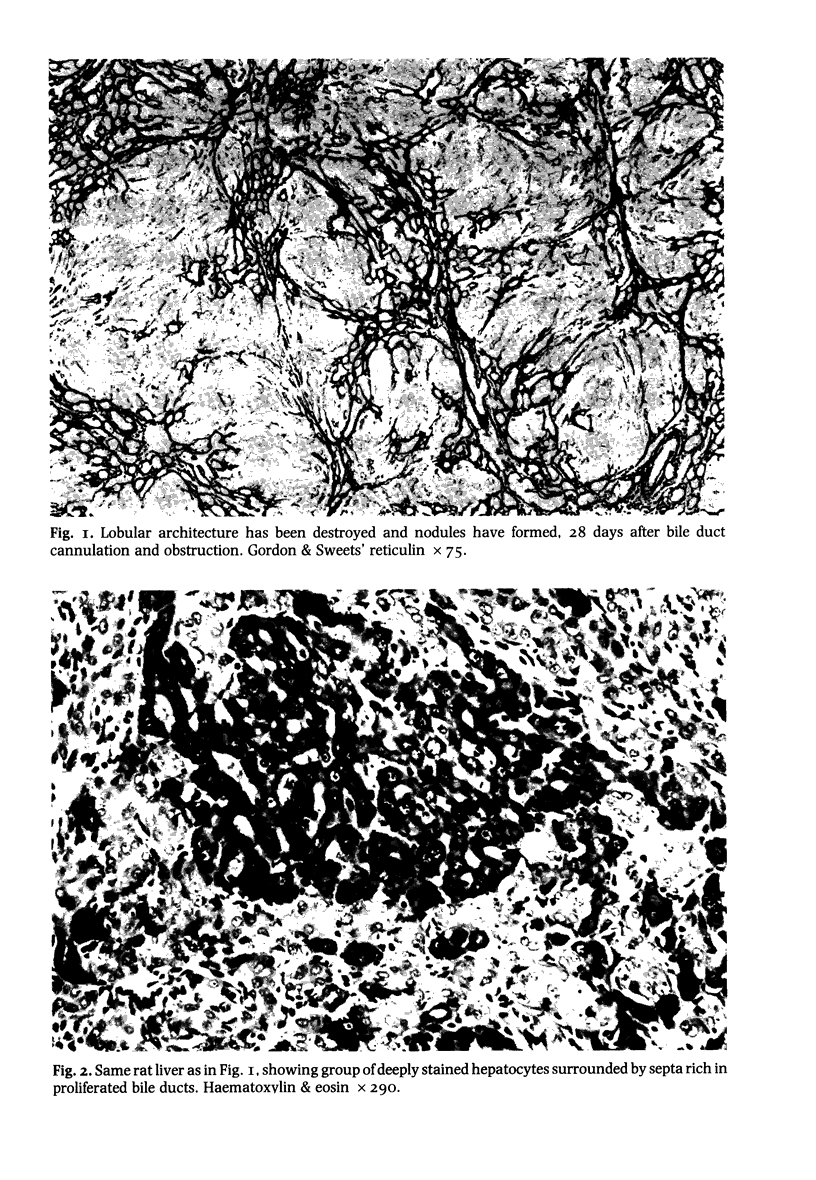
Hepatic morphological abnormalities were examined in rats whose bile ducts had been either cannulated and then obstructed or irreversibly ligated for 5, 10, 15 and 28 days or longer. Throughout the experiment most of the morphological changes observed in the cannulated group were comparable to those in the ligated group. Portal inflammation and marginal bile duct proliferation were noted with the same frequency in both groups. Biliary obstruction for 15 days or more led to cirrhosis. After 28 days obstruction, five out of six cannulated rats and four out of six ligated animals respectively developed cirrhosis. The development of cirrhosis was progressive and associated with ascites. It is concluded that in the rat the morphological sequelae of long term cholestasis induced by either cannulation and obstruction or ligation of bile ducts are similar and are accompanied by cirrhosis. The advantages of this experimental model for the study of human cirrhosis are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accatino L., Contreras A., Berdichevsky E., Quintana C. The effect of complete biliary obstruction on bile secretion. Studies on the mechanisms of postcholestatic choleresis in the rat. J Lab Clin Med. 1981 Apr;97(4):525–534. [PubMed] [Google Scholar]
- Accatino L., Contreras A., Fernańdez S., Quintana C. The effect of complete biliary obstruction on bile flow and bile acid excretion: postcholestatic choleresis in the rat. J Lab Clin Med. 1979 May;93(5):706–717. [PubMed] [Google Scholar]
- CAMERON G. R., MUZAFFAR HASAN S. Disturbances of structure and function in the liver as the result of biliary obstruction. J Pathol Bacteriol. 1958 Apr;75(2):333–349. doi: 10.1002/path.1700750212. [DOI] [PubMed] [Google Scholar]
- MICHAUELSSON M., NOSSLIN B., SJOELIN S. PLASMA BILIRUBIN DETERMINATION IN THE NEWBORN INFANT. A METHODOLOGICAL STUDY WITH SPECIAL REFERENCE TO THE INFLUENCE OF HEMOLYSIS. Pediatrics. 1965 Jun;35:925–931. [PubMed] [Google Scholar]
- Miura S., Asakura H., Munakata Y., Kobayashi K., Yoshioka M., Morishita T., Tsuchiya M. Lymphatic role in the pathogenesis of fat malabsorption in liver cirrhosis in rats. Dig Dis Sci. 1982 Nov;27(11):1030–1036. doi: 10.1007/BF01391751. [DOI] [PubMed] [Google Scholar]
- Moritz M., Snodgrass P. J. Serum enzymes derived from liver cell fractions. II. Responses to bile duct ligation in rats. Gastroenterology. 1972 Jan;62(1):93–100. [PubMed] [Google Scholar]
- Owen C. A. Copper metabolism after biliary fistula, obstruction, or sham operation in rats. Mayo Clin Proc. 1975 Jul;50(7):412–415. [PubMed] [Google Scholar]
- Proctor E., Chatamra K. Controlled induction of cirrhosis in the rat. Br J Exp Pathol. 1983 Jun;64(3):320–330. [PMC free article] [PubMed] [Google Scholar]
- Pérez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology. 1983 Jan-Feb;3(1):112–120. doi: 10.1002/hep.1840030118. [DOI] [PubMed] [Google Scholar]