Abstract
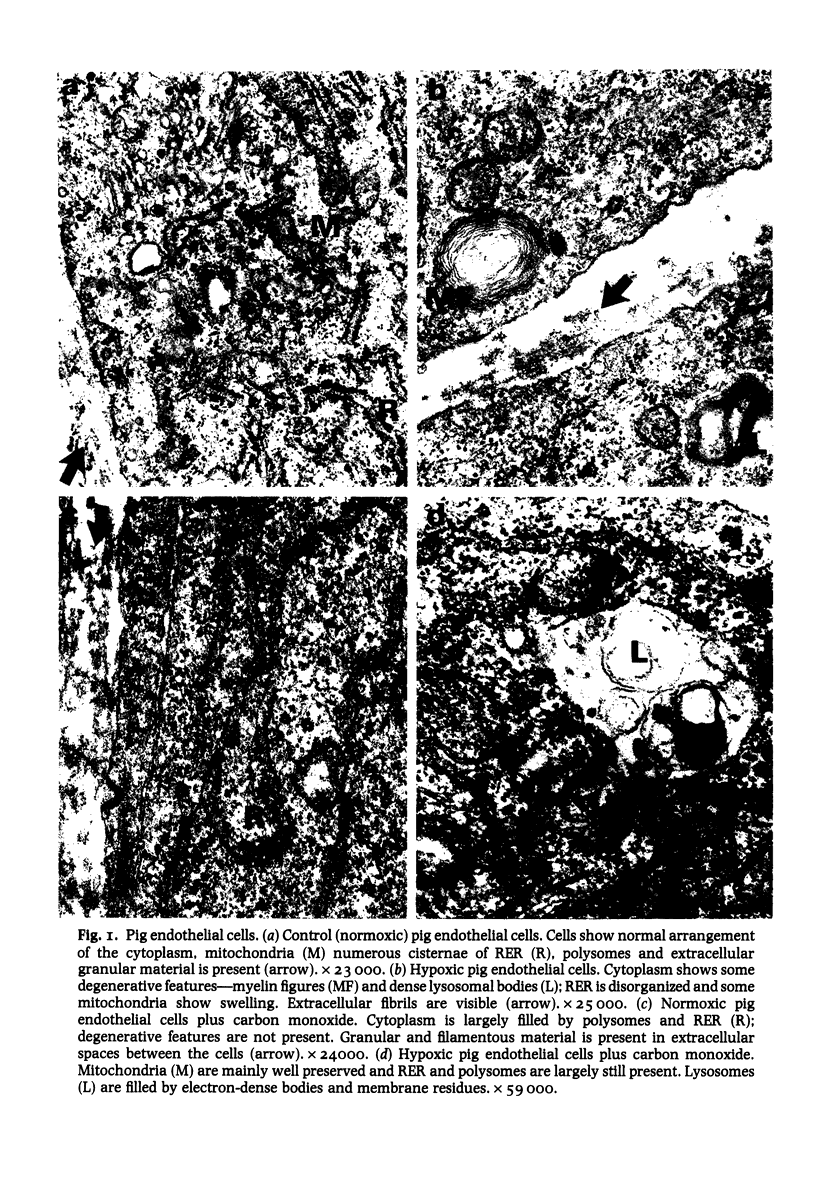
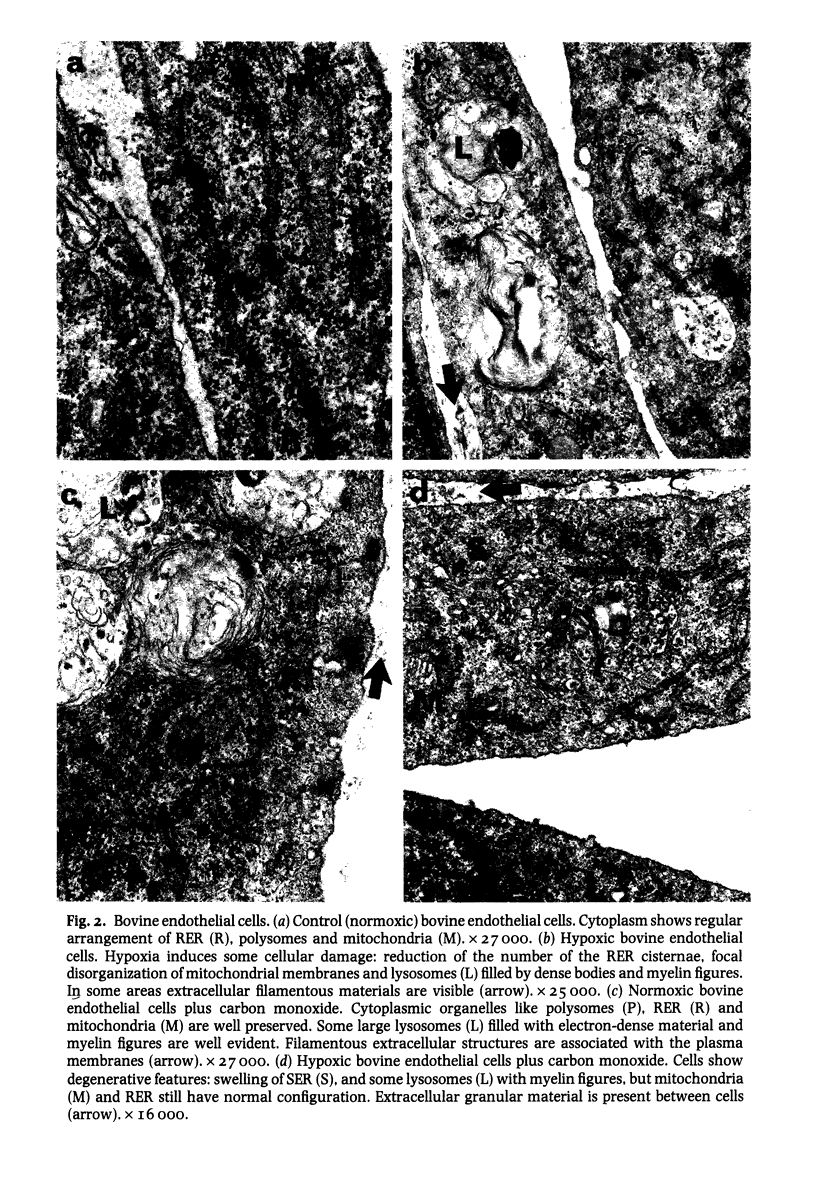
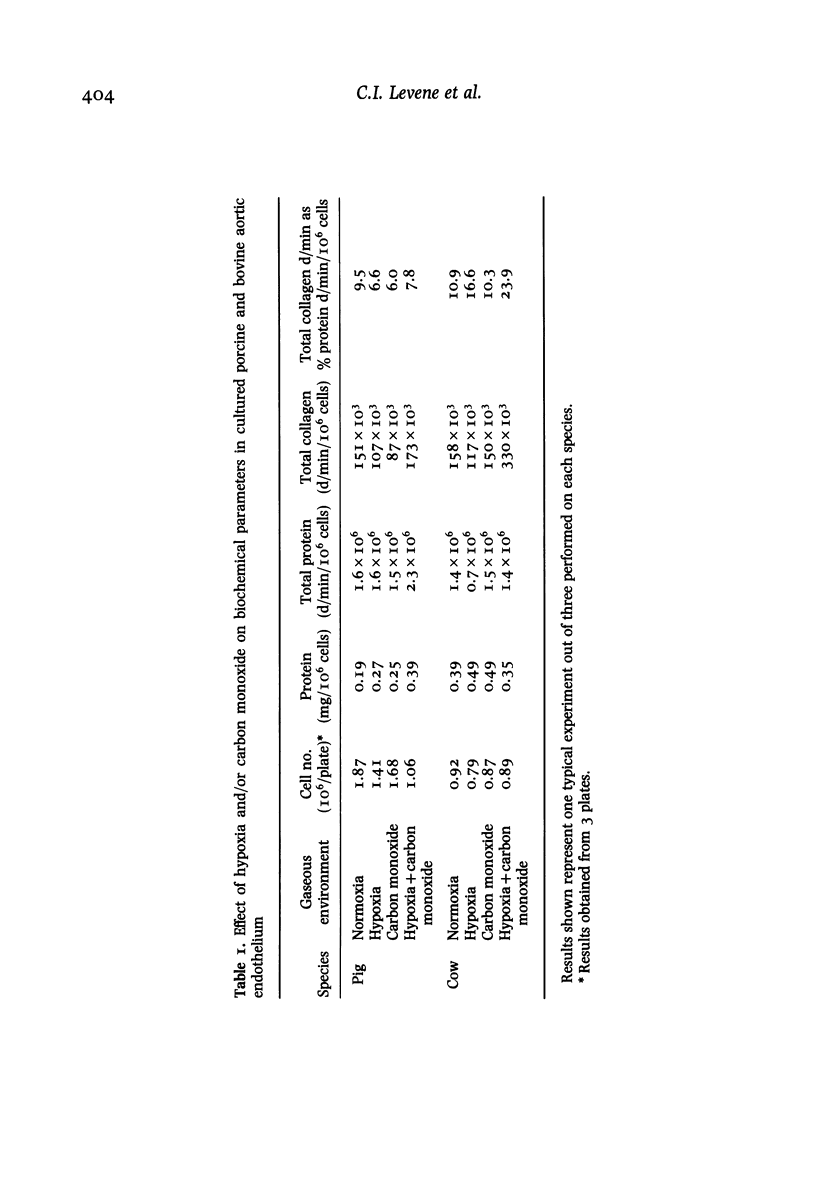
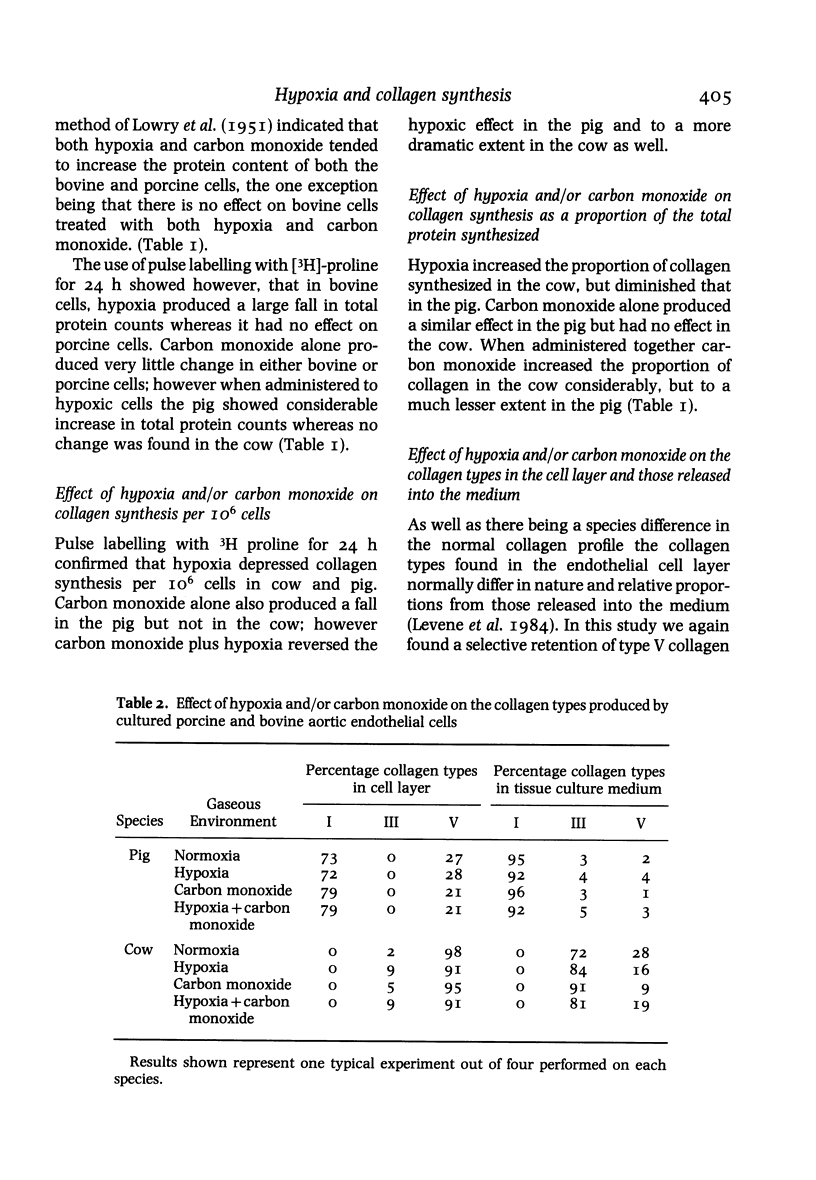
The cell layers and medium of cultured porcine and bovine aortic endothelium have been examined to test the effects of 24 h treatment with two factors associated with cigarette smoke--hypoxia and carbon monoxide, on cell numbers, total protein including collagen/10(6) cells, collagen type profile and ultrastructure. The most significant findings were that the responses varied with the species and that the effects on protein synthesis including collagen differed depending on the nature of the insult; in general, moreover carbon monoxide tended to reverse the action of hypoxia, a finding supported by ultrastructural evidence. The phenotypic collagen profiles were unaffected by either hypoxia or carbon monoxide.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrup P., Kjeldsen K., Wanstrup J. Effects of carbon monoxide exposure on the arterial walls. Ann N Y Acad Sci. 1970 Oct 5;174(1):294–300. doi: 10.1111/j.1749-6632.1970.tb49796.x. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M. J., Morton L. F., Levene C. I. Synthesis of interstitial collagens by pig aortic endothelial cells in culture. Biochem Biophys Res Commun. 1978 Oct 16;84(3):646–653. doi: 10.1016/0006-291x(78)90754-4. [DOI] [PubMed] [Google Scholar]
- Bewley B. R. Smoking in pregnancy. Br Med J (Clin Res Ed) 1984 Feb 11;288(6415):424–426. doi: 10.1136/bmj.288.6415.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leake D. S., Bowyer D. E. Quantitative studies of pinocytosis by arterial endothelial and smooth muscle cells in culture. Exp Mol Pathol. 1981 Aug;35(1):84–97. doi: 10.1016/0014-4800(81)90009-5. [DOI] [PubMed] [Google Scholar]
- Levene C. I., Bartlet C., Heale G. Phenotypic changes in morphology and collagen polymorphism of cultured bovine and porcine aortic endothelium. Atherosclerosis. 1984 Jul;52(1):59–71. doi: 10.1016/0021-9150(84)90156-4. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Dreyer B., Pitlick F. A., Furthmayr H. The collagenous components of the subendothelium. Correlation of structure and function. Lab Invest. 1980 Oct;43(4):303–315. [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]