Abstract
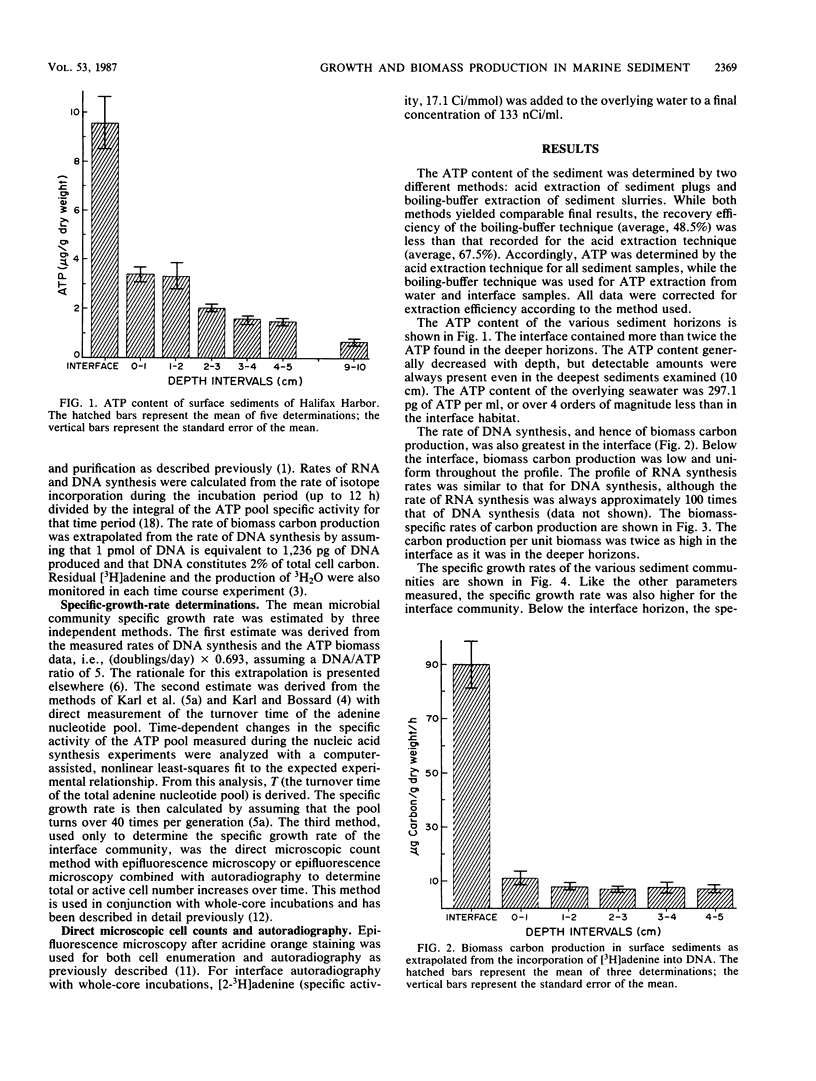
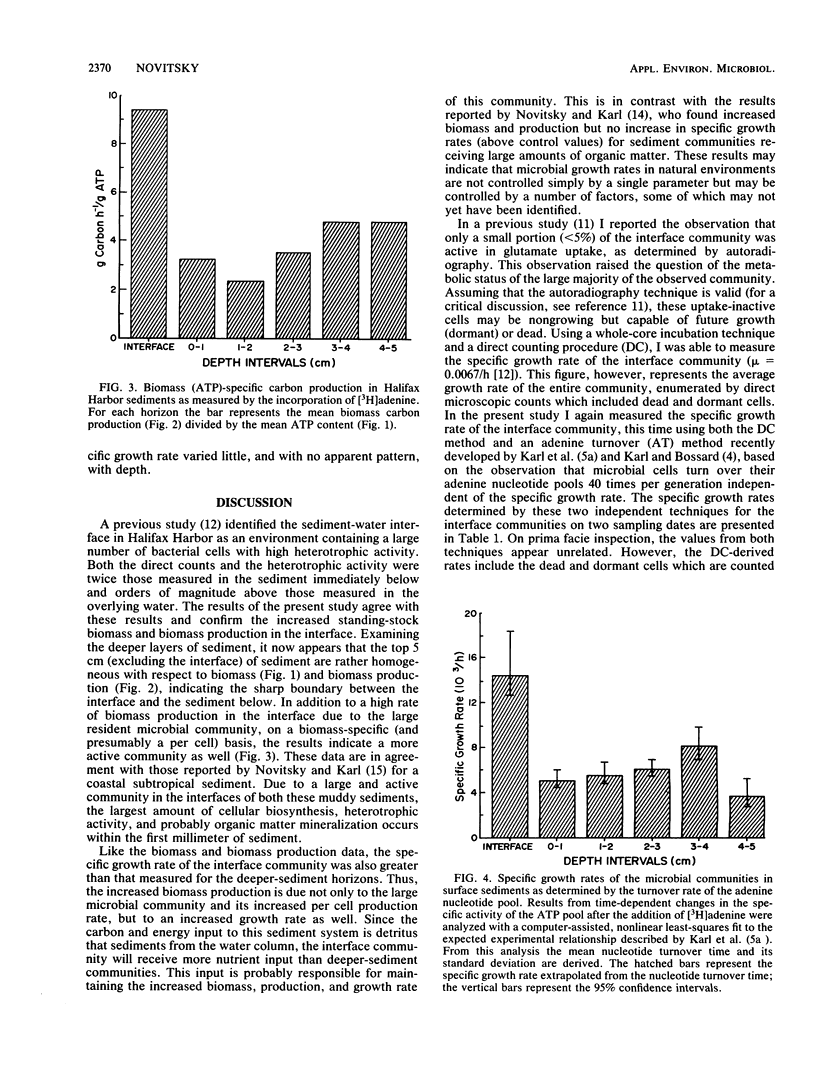
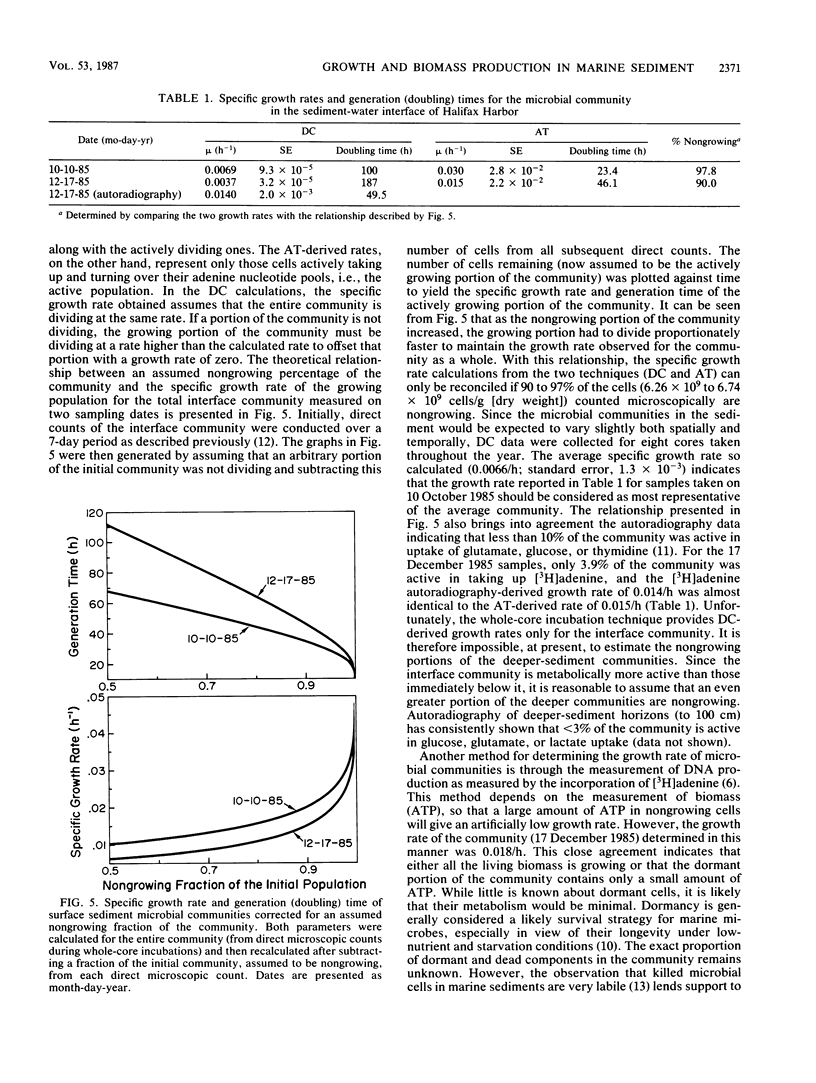
Biomass, nucleic acid synthesis, and specific growth rates of the microbial communities were measured throughout a vertical profile of a coastal marine sediment. The microbial biomass, as determined by ATP concentrations, in the sediment-water interface was over twice that measured in the other horizons of a 10-cm profile. Likewise, biomass carbon production, as determined by DNA synthesis, and the specific growth rate, as determined from the kinetics of [3H]ATP pool labeling, were also elevated at the interface. These results indicate that, due to a large and active community in the interface, the greatest amount of microbial activity, growth, and biosynthesis occurs within the first few millimeters of sediment. These results notwithstanding, a combination of two independent techniques established that over 90% of the sediment-water interface community was not actively growing.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Karl D. M., Craven D. B. Effects of alkaline phosphatase activity on nucleotide measurements in aquatic microbial communities. Appl Environ Microbiol. 1980 Sep;40(3):549–561. doi: 10.1128/aem.40.3.549-561.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D. M. Simultaneous rates of ribonucleic Acid and deoxyribonucleic Acid syntheses for estimating growth and cell division of aquatic microbial communities. Appl Environ Microbiol. 1981 Nov;42(5):802–810. doi: 10.1128/aem.42.5.802-810.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepkay P. E., Cooke R. C., Novitsky J. A. Microbial autotrophy: a primary source of organic carbon in marine sediments. Science. 1979 Apr 6;204(4388):68–69. doi: 10.1126/science.204.4388.68. [DOI] [PubMed] [Google Scholar]
- Meyer-Reil L. A. Autoradiography and epifluorescence microscopy combined for the determination of number and spectrum of actively metabolizing bacteria in natural water. Appl Environ Microbiol. 1978 Sep;36(3):506–512. doi: 10.1128/aem.36.3.506-512.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A. Degradation of dead microbial biomass in a marine sediment. Appl Environ Microbiol. 1986 Sep;52(3):504–509. doi: 10.1128/aem.52.3.504-509.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A. Heterotrophic activity throughout a vertical profile of seawater and sediment in halifax harbor, Canada. Appl Environ Microbiol. 1983 Jun;45(6):1753–1760. doi: 10.1128/aem.45.6.1753-1760.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A., Karl D. M. Influence of deep ocean sewage outfalls on the microbial activity of the surrounding sediment. Appl Environ Microbiol. 1985 Dec;50(6):1464–1473. doi: 10.1128/aem.50.6.1464-1473.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A. Microbial activity at the sediment-water interface in halifax harbor, Canada. Appl Environ Microbiol. 1983 Jun;45(6):1761–1766. doi: 10.1128/aem.45.6.1761-1766.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor P. S., Neihof R. A. Improved microautoradiographic method to determine individual microorganisms active in substrate uptake in natural waters. Appl Environ Microbiol. 1982 Oct;44(4):945–953. doi: 10.1128/aem.44.4.945-953.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn C. D., Karl D. M. Laboratory calibrations of the [h]adenine technique for measuring rates of RNA and DNA synthesis in marine microorganisms. Appl Environ Microbiol. 1984 Apr;47(4):835–842. doi: 10.1128/aem.47.4.835-842.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R., Iturriaga R., Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978 Dec;36(6):926–935. doi: 10.1128/aem.36.6.926-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


