Abstract
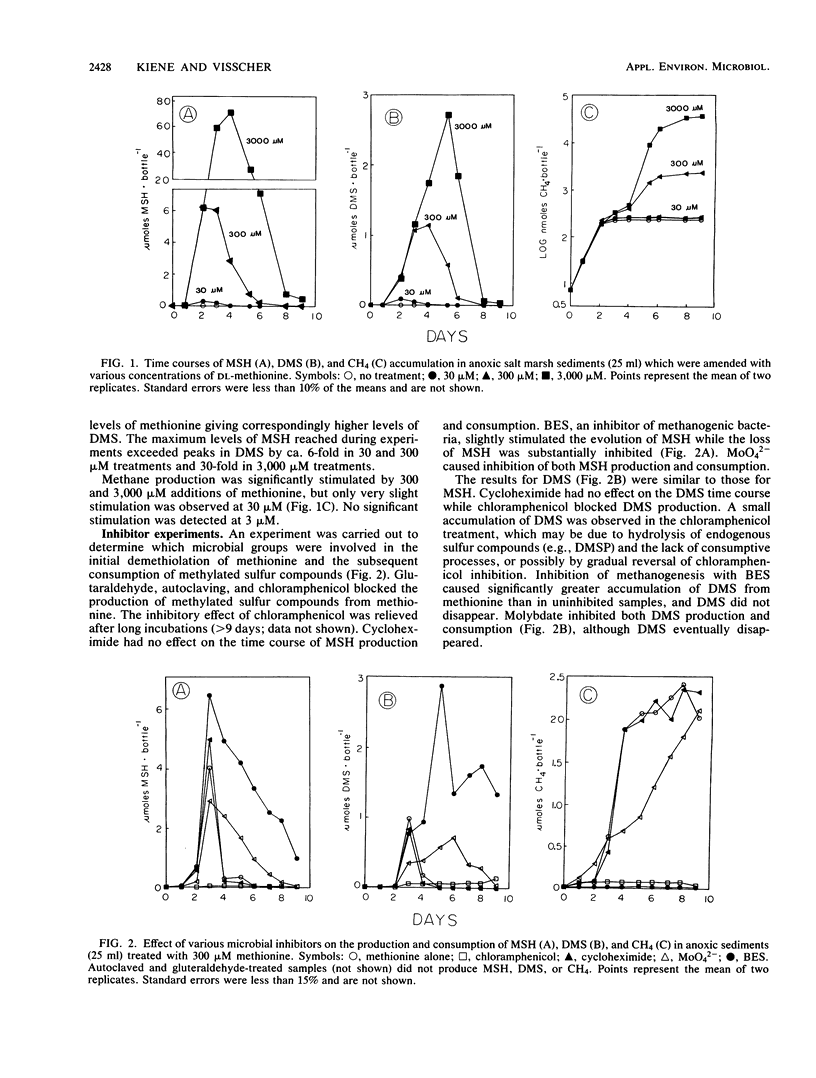
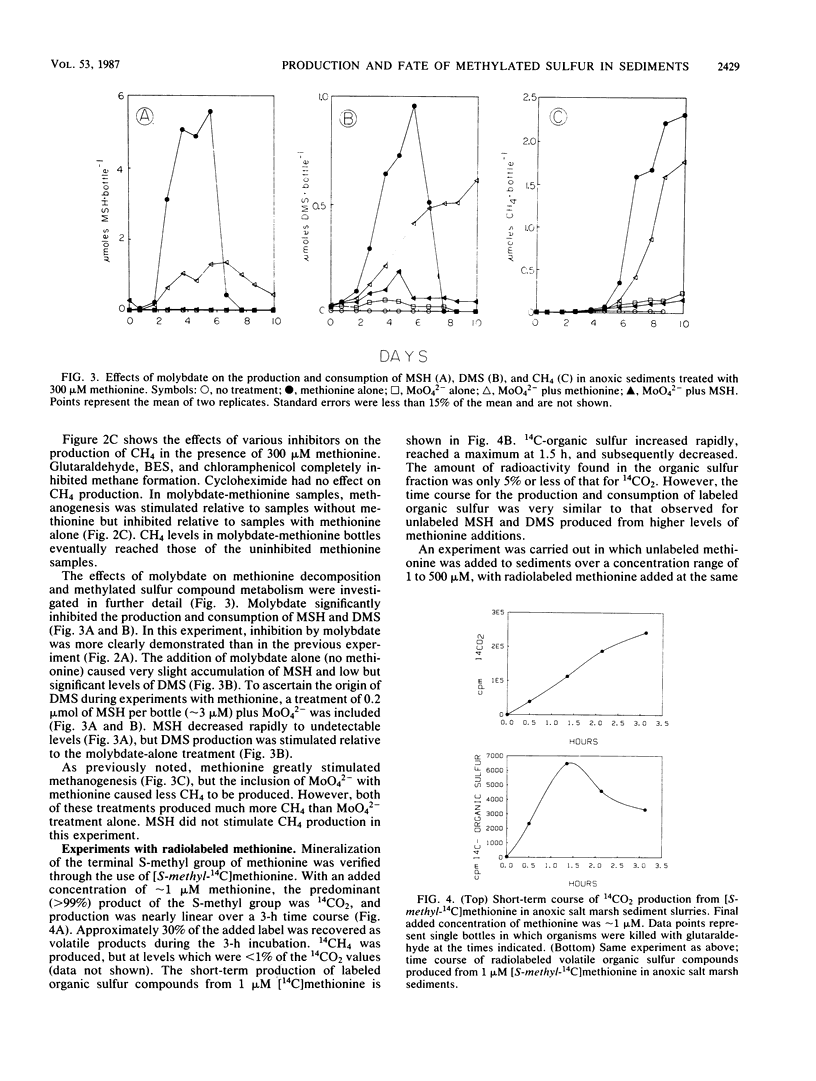
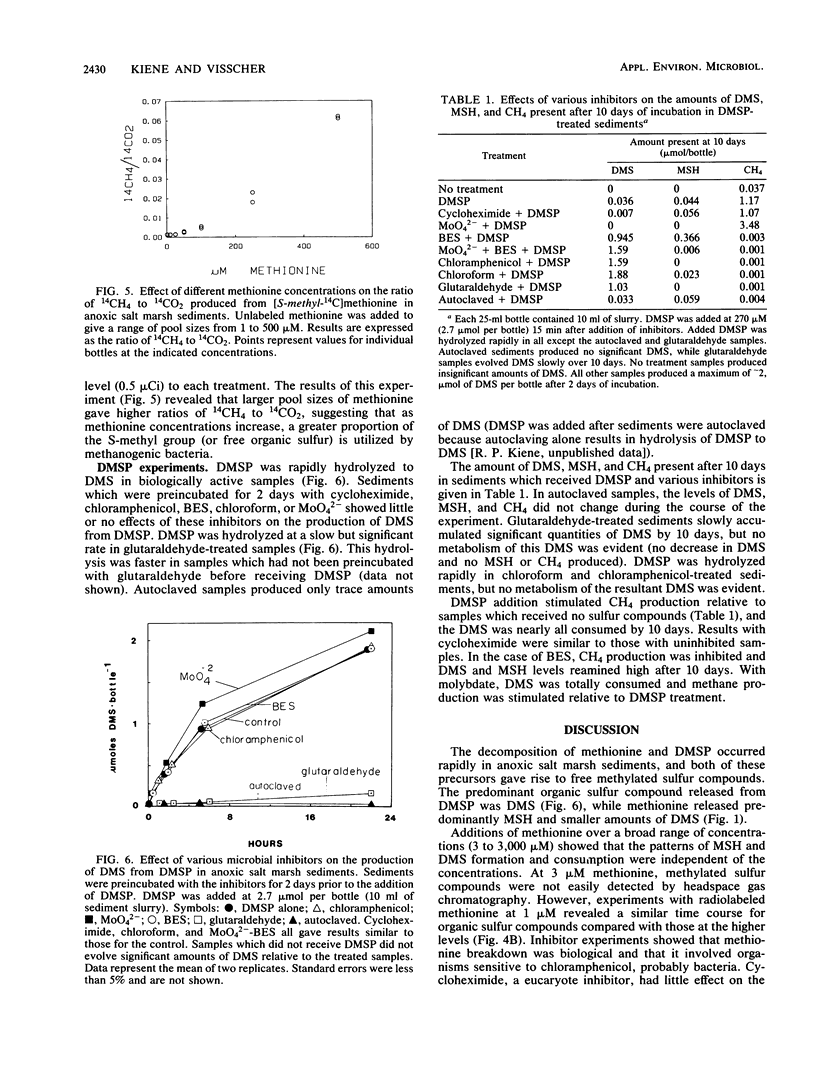
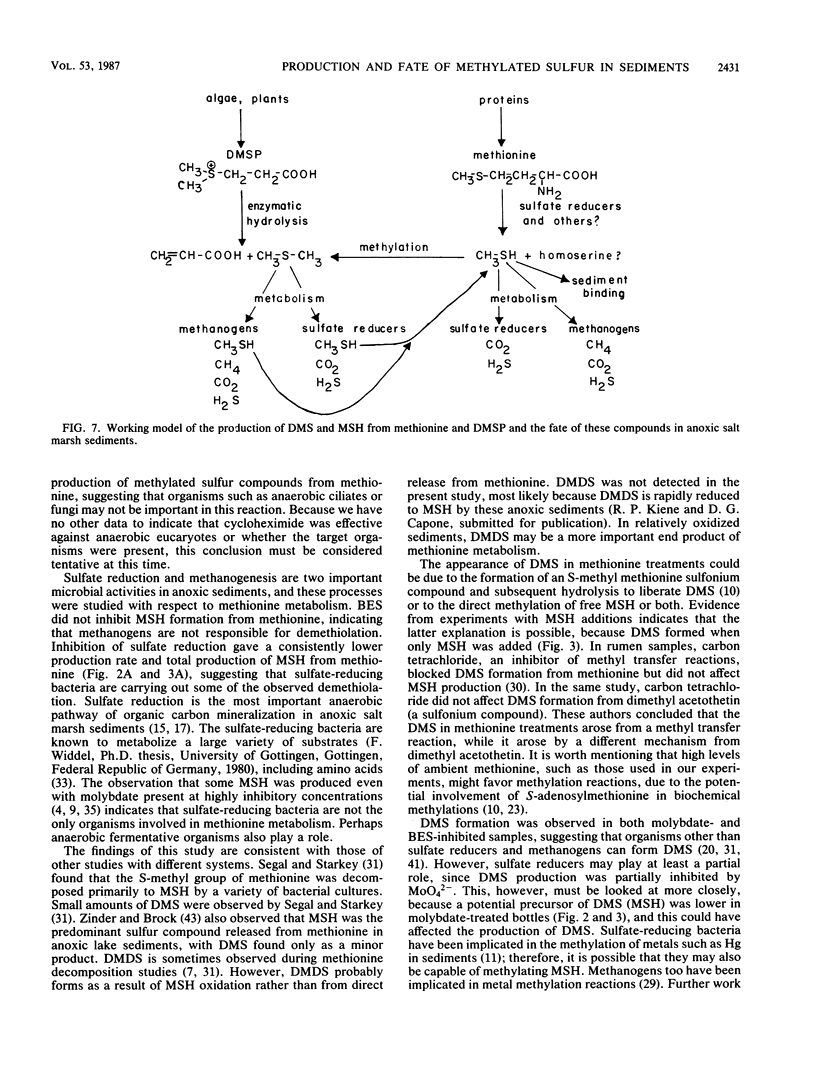
Anoxic salt marsh sediments were amended with dl-methionine and dimethylsulfoniopropionate (DMSP). Microbial metabolism of methionine yielded methane thiol (MSH) as the major volatile organosulfur product, with the formation of lesser amounts of dimethylsulfide (DMS). Biological transformation of DMSP resulted in the rapid release of DMS and only small amounts of MSH. Experiments with microbial inhibitors indicated that production of MSH from methionine was carried out by procaryotic organisms, probably sulfate-reducing bacteria. Methane-producing bacteria did not metabolize methionine. The involvement of specific groups of organisms in DMSP hydrolysis could not be determined with the inhibitors used, because DMSP was hydrolyzed in all samples except those which were autoclaved. Unamended sediment slurries, prepared from Spartina alterniflora sediments, contained significant (1 to 10 μM) concentrations of DMS. Endogenous methylated sulfur compounds and those produced from added methionine and DMSP were consumed by sediment microbes. Both sulfate-reducing and methane-producing bacteria were involved in DMS and MSH consumption. Methanogenesis was stimulated by the volatile organosulfur compounds released from methionine and DMSP. However, apparent competition for these compounds exists between methanogens and sulfate reducers. At low (1 μM) concentrations of methionine, the terminal S-methyl group was metabolized almost exclusively to CO2 and only small amounts of CH4. At higher (>100 μM) concentrations of methionine, the proportion of the methyl-sulfur group converted to CH4 increased. The results of this study demonstrate that methionine and DMSP are potential precursors of methylated sulfur compounds in anoxic sediments and that the microbial community is capable of metabolizing volatile methylated sulfur compounds.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON D. G., CANTONI G. L. Enzymatic cleavage of dimethylpropiothetin by Polysiphonia lanosa. J Biol Chem. 1956 Sep;222(1):171–177. [PubMed] [Google Scholar]
- Bauchop T. Inhibition of rumen methanogenesis by methane analogues. J Bacteriol. 1967 Jul;94(1):171–175. doi: 10.1128/jb.94.1.171-175.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone D. G., Reese D. D., Kiene R. P. Effects of metals on methanogenesis, sulfate reduction, carbon dioxide evolution, and microbial biomass in anoxic salt marsh sediments. Appl Environ Microbiol. 1983 May;45(5):1586–1591. doi: 10.1128/aem.45.5.1586-1591.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compeau G. C., Bartha R. Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Appl Environ Microbiol. 1985 Aug;50(2):498–502. doi: 10.1128/aem.50.2.498-502.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Romesser J. A., Wolfe R. S. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978 Jun 13;17(12):2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- Kiene R. P., Oremland R. S., Catena A., Miller L. G., Capone D. G. Metabolism of reduced methylated sulfur compounds in anaerobic sediments and by a pure culture of an estuarine methanogen. Appl Environ Microbiol. 1986 Nov;52(5):1037–1045. doi: 10.1128/aem.52.5.1037-1045.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso S. The relationship between methionine uptake and demethiolation in a methionine-utilizing mutant of Pseudomonas fluorescens UK1. J Gen Microbiol. 1976 Aug;96(2):391–394. doi: 10.1099/00221287-95-2-391. [DOI] [PubMed] [Google Scholar]
- Oremland R. S., Marsh L., Desmarais D. J. Methanogenesis in big soda lake, nevada: an alkaline, moderately hypersaline desert lake. Appl Environ Microbiol. 1982 Feb;43(2):462–468. doi: 10.1128/aem.43.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Polcin S. Methanogenesis and sulfate reduction: competitive and noncompetitive substrates in estuarine sediments. Appl Environ Microbiol. 1982 Dec;44(6):1270–1276. doi: 10.1128/aem.44.6.1270-1276.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley W. P., Dizikes L. J., Wood J. M. Biomethylation of toxic elements in the environment. Science. 1977 Jul 22;197(4301):329–332. doi: 10.1126/science.877556. [DOI] [PubMed] [Google Scholar]
- Segal W., Starkey R. L. Microbial decomposition of methionine and identity of the resulting sulfur products. J Bacteriol. 1969 Jun;98(3):908–913. doi: 10.1128/jb.98.3.908-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocher C. S., Ackman R. G., McLachlan J. The identification of dimethyl-beta-propiothetin in the algae syracosphaera Carterae and Ulva lactuca. Can J Biochem. 1966 May;44(5):519–522. doi: 10.1139/o66-062. [DOI] [PubMed] [Google Scholar]
- WAGNER C., STADTMAN E. R. Bacterial fermentation of dimethyl-beta-propiothetin. Arch Biochem Biophys. 1962 Aug;98:331–336. doi: 10.1016/0003-9861(62)90191-1. [DOI] [PubMed] [Google Scholar]
- Winfrey M. R., Ward D. M. Substrates for sulfate reduction and methane production in intertidal sediments. Appl Environ Microbiol. 1983 Jan;45(1):193–199. doi: 10.1128/aem.45.1.193-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Growth of Rhodopseudomonas capsulata under anaerobic dark conditions with dimethyl sulfoxide. Arch Biochem Biophys. 1977 Jun;181(2):411–418. doi: 10.1016/0003-9861(77)90246-6. [DOI] [PubMed] [Google Scholar]
- Zinder S. H., Brock T. D. Methane, carbon dioxide, and hydrogen sulfide production from the terminal methiol group of methionine by anaerobic lake sediments. Appl Environ Microbiol. 1978 Feb;35(2):344–352. doi: 10.1128/aem.35.2.344-352.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Doemel W. N., Brock T. D. Production of volatile sulfur compounds during the decomposition of algal mats. Appl Environ Microbiol. 1977 Dec;34(6):859–860. doi: 10.1128/aem.34.6.859-860.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



