Abstract
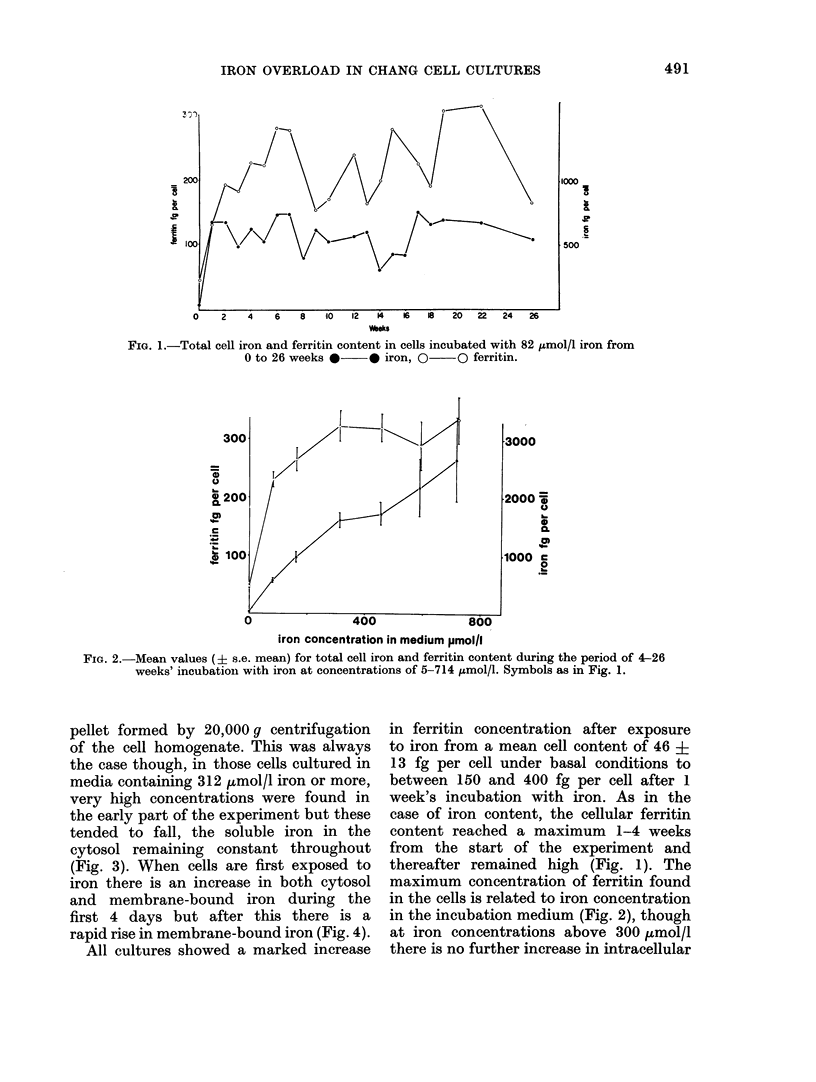
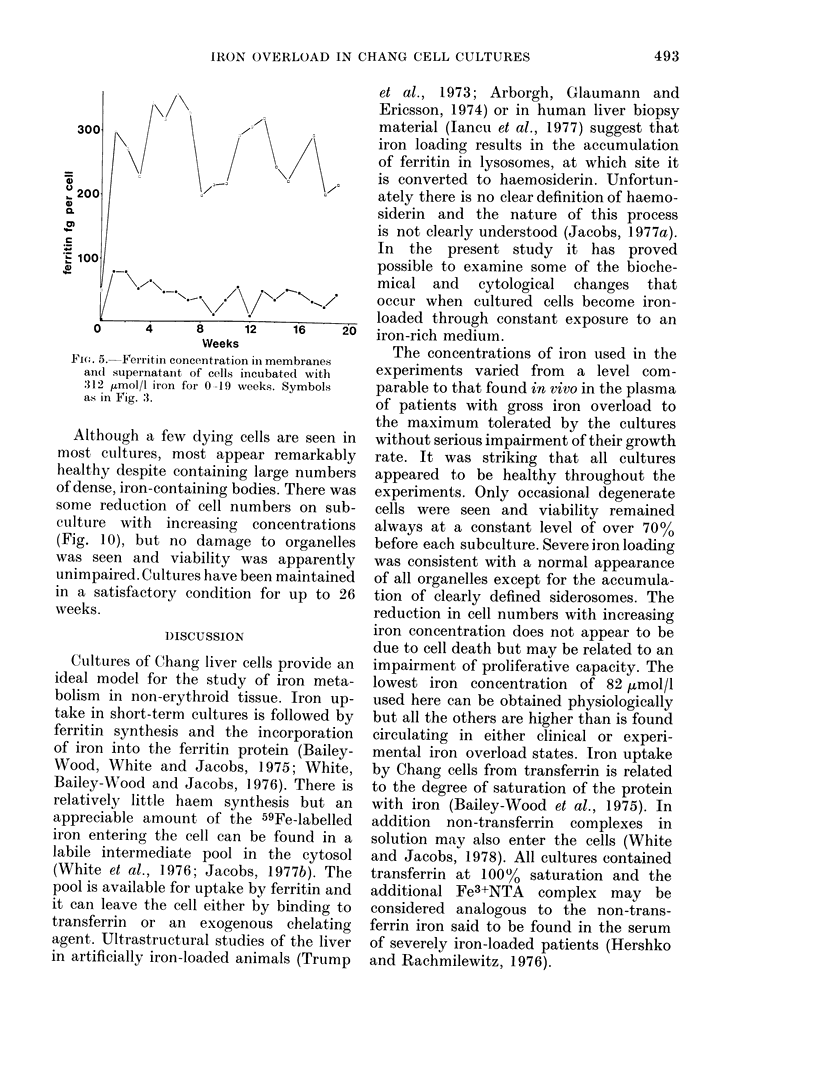
Cultures of Chang cells have been studied during growth in media supplemented with ferric nitriloacetate. Iron loading of the cells occurs rapidly and is related to the iron concentration in the medium. A 50-fold increase in cellular iron content was obtained in some cultures. Most of the intracellular iron is membrane-bound and is seen on electron microscopy to be concentrated in discrete bodies. There is a rapid rise in cellular ferritin content after exposure to iron. Most of this is found in the cytosol.
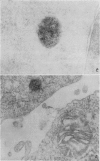
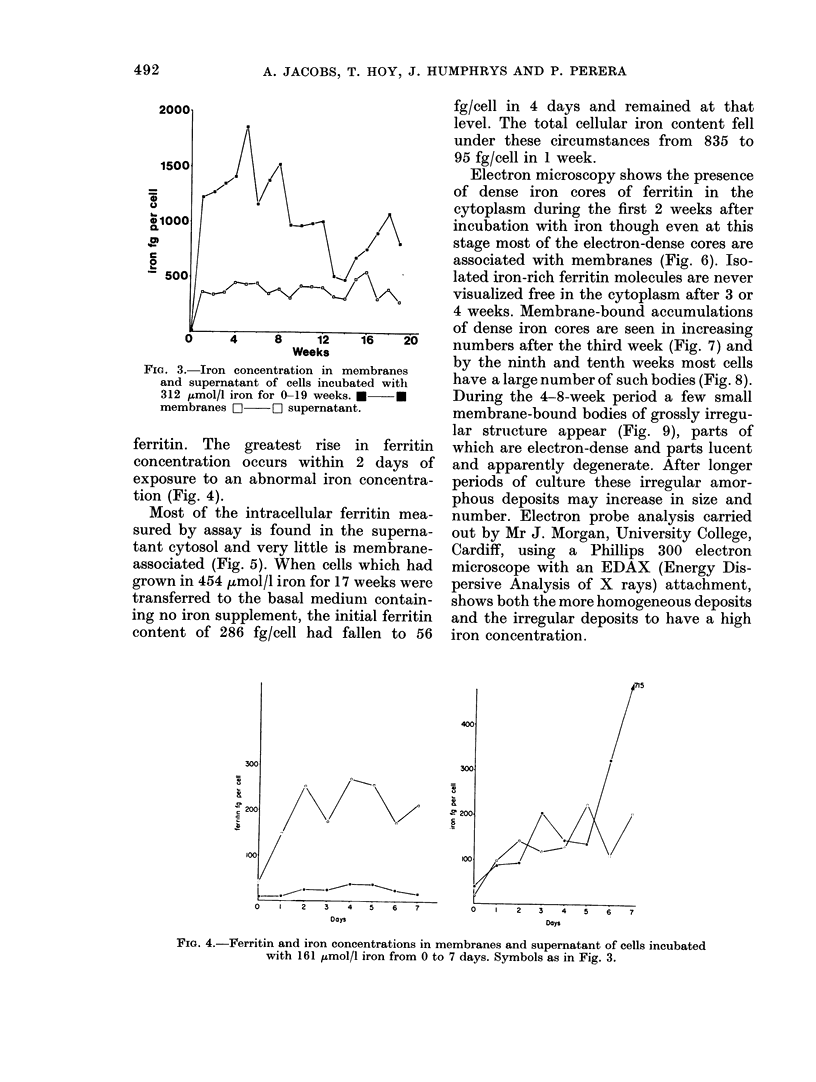
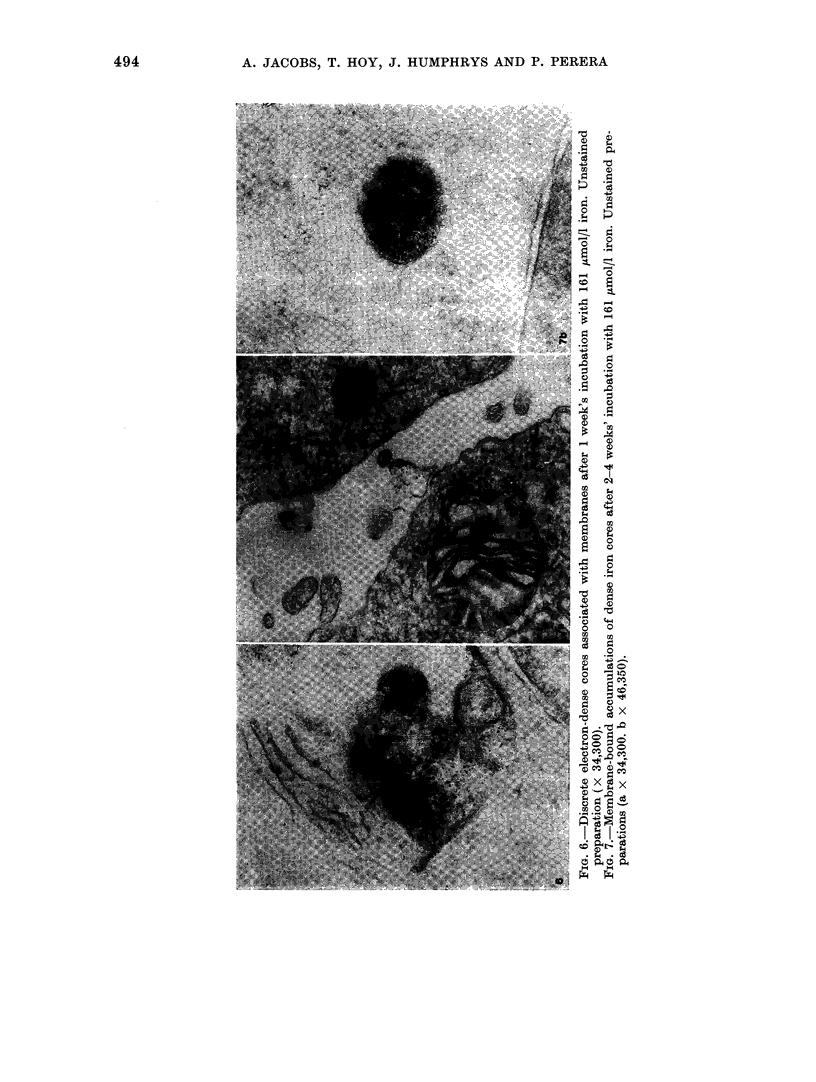
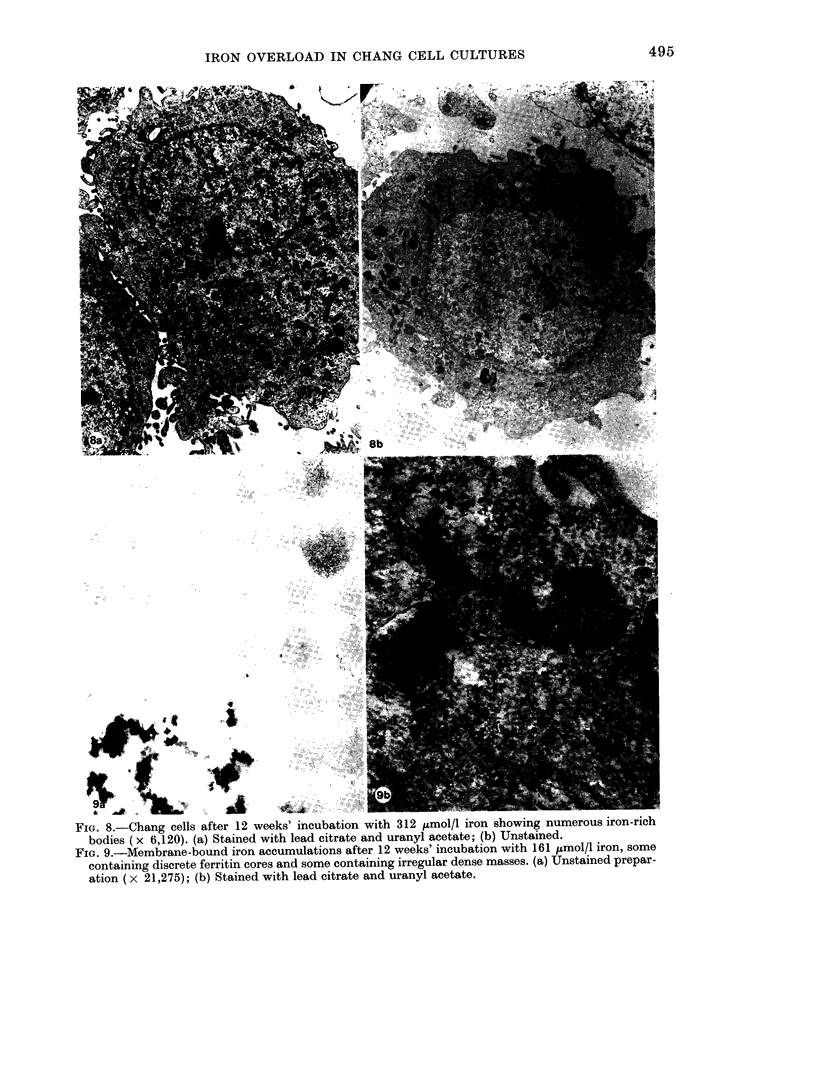
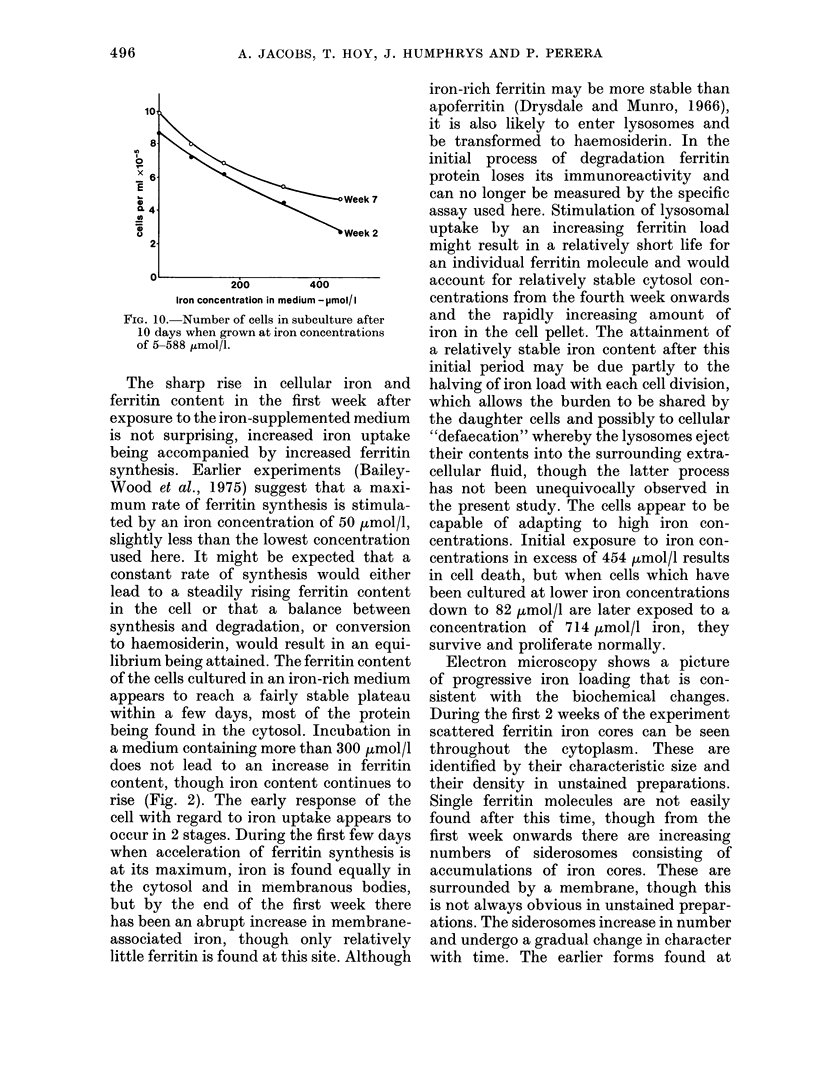
Iron taken into the cells is found equally in the cytosol and associated with membranes for the first 4 days of culture. After this time there is a rapid rise of membrane-bound iron associated with the formation of siderosomes which contain iron-rich ferritin cores. These siderosomes later evolve to contain irregular, electron-dense accumulations of iron.
Initial exposure of cells to high iron concentrations causes rapid death but similar exposure after ferritin synthesis and siderosome formation has been stimulated by low iron concentrations is well tolerated. Cultures have been maintained for up to 26 weeks with no morphological signs of toxicity, though there is some impairment of proliferation at high iron concentrations. It is suggested that siderosome formation is part of the mechanism that protects the cell against iron toxicity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arborgh B. A., Glaumann H., Ericsson J. L. Studies on iron loading of rat liver lysosomes. Effects on the liver and distribution and fate of iron. Lab Invest. 1974 May;30(5):664–673. [PubMed] [Google Scholar]
- Bailey-Wood R., White G. P., Jacobs A. The use of Chang cells cultured in vitro for the investigation of cellular iron metabolism. Br J Exp Pathol. 1975 Aug;56(4):358–362. [PMC free article] [PubMed] [Google Scholar]
- Barry M., Flynn D. M., Letsky E. A., Risdon R. A. Long-term chelation therapy in thalassaemia major: effect on liver iron concentration, liver histology, and clinical progress. Br Med J. 1974 Apr 6;2(5909):16–20. doi: 10.1136/bmj.2.5909.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem. 1966 Aug 10;241(15):3630–3637. [PubMed] [Google Scholar]
- Fischbach F. A., Gregory D. W., Harrison P. M., Hoy T. G., Williams J. M. On the structure of hemosiderin and its relationship to ferritin. J Ultrastruct Res. 1971 Dec;37(5):495–503. doi: 10.1016/s0022-5320(71)80020-5. [DOI] [PubMed] [Google Scholar]
- GOLBERG L., SMITH J. P., MARTIN L. E. The effects of intensive and prolonged administration of iron parenterally in animals. Br J Exp Pathol. 1957 Jun;38(3):297–311. [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977 Sep;50(3):433–439. [PubMed] [Google Scholar]
- Jones B. M., Worwood M. An automated immunoradiometric assay for ferritin. J Clin Pathol. 1975 Jul;28(7):540–542. doi: 10.1136/jcp.28.7.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell B. Management of thalassaemia major. Br Med Bull. 1976 Sep;32(3):270–276. doi: 10.1093/oxfordjournals.bmb.a071374. [DOI] [PubMed] [Google Scholar]
- Niitsu Y., Listowsky I. Mechanisms for the formation of ferritin oligomers. Biochemistry. 1973 Nov 6;12(23):4690–4695. doi: 10.1021/bi00747a023. [DOI] [PubMed] [Google Scholar]
- OLIVER R. A. Siderosis following transfusions of blood. J Pathol Bacteriol. 1959 Jan;77(1):171–194. doi: 10.1002/path.1700770118. [DOI] [PubMed] [Google Scholar]
- Pechet G. S. Parenteral iron overload. Organ and cell distribution in rats. Lab Invest. 1969 Jan;20(1):119–126. [PubMed] [Google Scholar]
- Peters T. J., Seymour C. A. Acid hydrolase activities and lysosomal integrity in liver biopsies from patients with iron overload. Clin Sci Mol Med. 1976 Jan;50(1):75–78. doi: 10.1042/cs0500075. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Valigorsky J. M., Arstila A. U., Mergner W. J., Kinney T. D. The relationship of intracellular pathways of iron metabolism to cellular iron overload and the iron storage diseases. Cell sap and cytocavitary network pathways in relation to lysosomal storage and turnover of iron macromolecules. Am J Pathol. 1973 Aug;72(2):295–336. [PMC free article] [PubMed] [Google Scholar]
- White G. P., Bailey-Wood R., Jacobs A. The effect of chelating agents on cellular iron metabolism. Clin Sci Mol Med. 1976 Mar;50(3):145–152. doi: 10.1042/cs0500145. [DOI] [PubMed] [Google Scholar]
- Williams M. A., Harrison P. M. Electron-microscopic and chemical studies of oligomers in horse ferritin. Biochem J. 1968 Nov;110(2):265–280. doi: 10.1042/bj1100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. The role of non-haem iron. Biochem J. 1969 Jun;113(2):325–332. doi: 10.1042/bj1130325. [DOI] [PMC free article] [PubMed] [Google Scholar]