Abstract
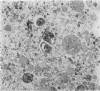
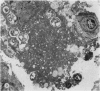
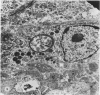
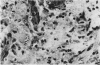
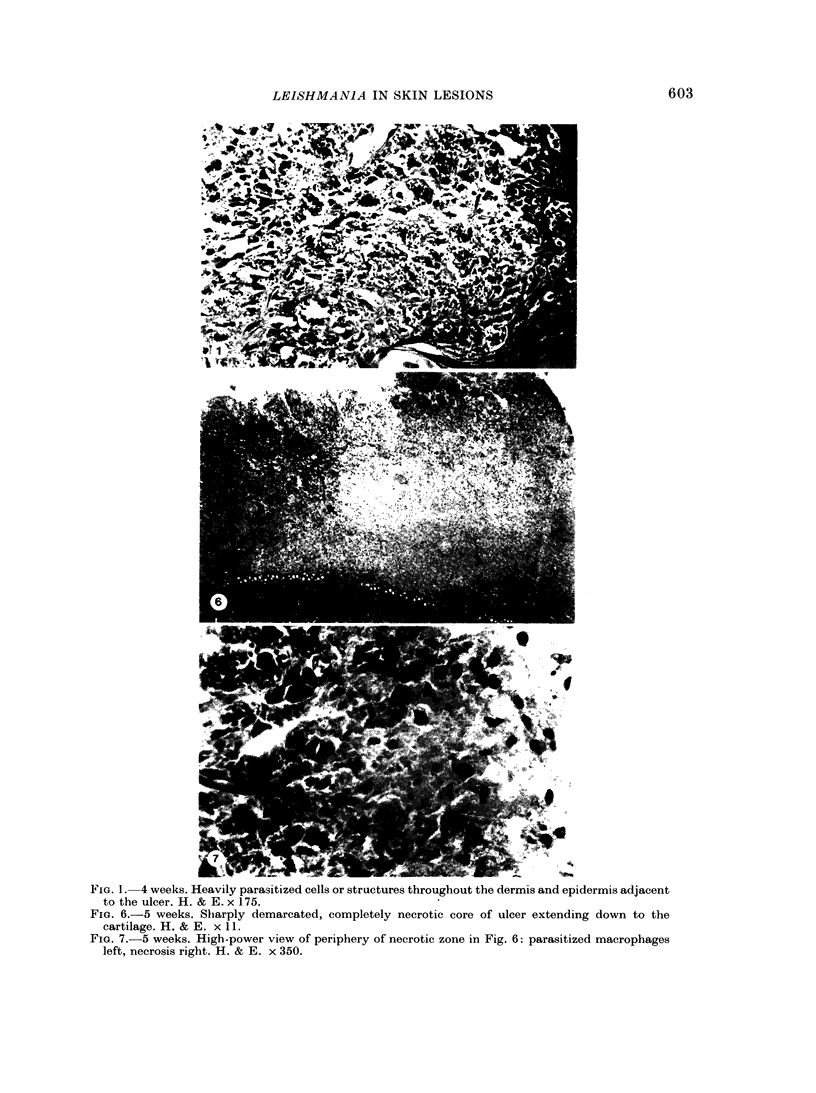
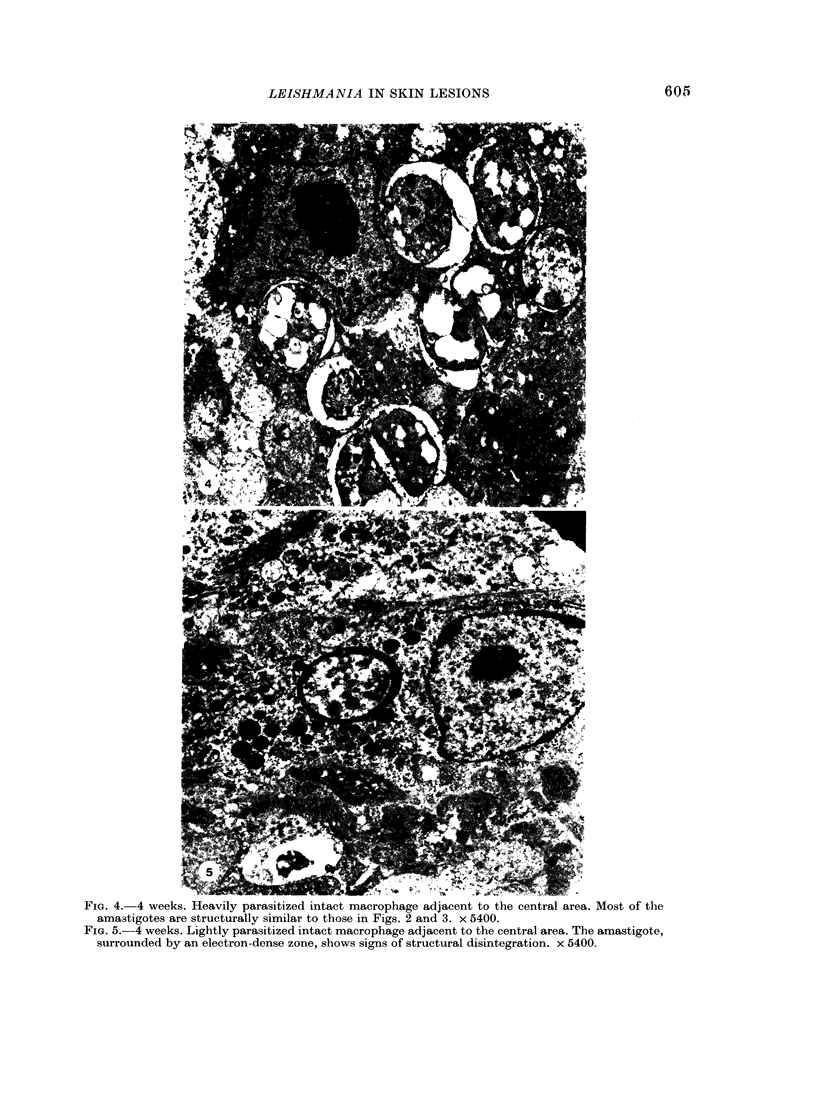
Nineteen guinea-pigs were each inoculated intradermally with 10(6) amastigotes of Leishmania enriettii, and the development of the lesions was followed from Weeks 4 to 10 with a view to elucidating the histological mechanisms involved with the elimination of parasites. Electron microscopic observations were made in 1 animal. Extensive necrosis of the parasite-laden macrophages was observed in 7 out of 7 animals at 4 and 5 weeks. In the ulcerated core of the lesion at 4 weeks no intact macrophages could be identified. Very many amastigotes were extracellular. Others were present in the cytoplasm of residual macrophages the cell walls of which had disintegrated. Necrosis was less marked at 8 weeks and absent in the resolving lesions at 10 weeks. Signs of stimulation or maturation of macrophages were only apparent when parasites were few. At 4 weeks macrophages were almost all of the non-stimulated form, but cytological evidence of activation became progressively more definite and widespread from 5 to 8 weeks, starting at the periphery of the lesion. Ultrastructural observations of amastigotes suggested that there might be more than one mechanism of degradation. It appeared that the majority of parasites were released through necrosis and discharged through the ulcer, and that intracellular degradation of the remaining parasites was important mainly in the later phase before resolution. The first phase was associated mainly with plasma-cell production, the second mainly with lymphocytes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The structure of mononuclear phagocytes differentiating in vivo. I. Sequential fine and histologic studies of the effect of Bacillus Calmette-Guerin (BCG). Am J Pathol. 1974 Jul;76(1):17–48. [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D., Bray R. S., Wolstencroft R. A., Dumonde D. C. Immunity in cutaneous leishmaniasis of the guinea-pig. Clin Exp Immunol. 1970 Sep;7(3):301–341. [PMC free article] [PubMed] [Google Scholar]
- Gardener P. J. Taxonomy of the genus Leishmania: a review of nomenclature and classification. Trop Dis Bull. 1977 Dec;74(12):1069–1088. [PubMed] [Google Scholar]
- Kanan M. W. Mucocutaneous leishmaniasis in guinea-pigs inoculated intravenously with Leishmania enriettii. Preliminary report. Br J Dermatol. 1975 Jun;92(6):663–673. doi: 10.1111/j.1365-2133.1975.tb03147.x. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauel J., Behin R., Biroum-Noerjasin, Rowe D. S. Mechanisms of protective immunity in experimental cutaneous leishmaniasis of the guinea-pig. I. Lack of effects of immune lymphocytes and of activated macrophages. Clin Exp Immunol. 1975 May;20(2):339–350. [PMC free article] [PubMed] [Google Scholar]
- Radwanski Z. K., Bryceson A. D., Preston P. M., Dumonde D. C. Immunoflorescence studies of Leishmania enriettii infection in the guinea pig. Trans R Soc Trop Med Hyg. 1974;68(2):124–132. doi: 10.1016/0035-9203(74)90185-0. [DOI] [PubMed] [Google Scholar]
- Rezai H. R., Haghighi P., Ardehali S. Histological appearance of the site of inoculation and lymph nodes of guinea-pigs at various times after infection with Leishmania enriettii. Trans R Soc Trop Med Hyg. 1972;66(2):225–234. doi: 10.1016/0035-9203(72)90151-4. [DOI] [PubMed] [Google Scholar]
- Ridley D. S. A histological classification of cutaneous leishmaniasis and its geographical expression. Trans R Soc Trop Med Hyg. 1980;74(4):515–521. doi: 10.1016/0035-9203(80)90069-3. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Marsden P. D., Cuba C. C., Barreto A. C. A histological classification of mucocutaneous leishmaniasis in Brazil and its clinical evaluation. Trans R Soc Trop Med Hyg. 1980;74(4):508–514. doi: 10.1016/0035-9203(80)90068-1. [DOI] [PubMed] [Google Scholar]
- Ridley D. S. The pathogenesis of cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1979;73(2):150–160. doi: 10.1016/0035-9203(79)90199-8. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Polak L., Parker D. Control mechanisms in delayed-type hypersensitivity. Br Med Bull. 1976 May;32(2):165–170. doi: 10.1093/oxfordjournals.bmb.a071350. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Nomoto K., Himeno K. Dissociation of erythema and basophil accumulation in Jones-Mote type hypersensitivity in the guinea-pig. Br J Exp Pathol. 1979 Dec;60(6):596–603. [PMC free article] [PubMed] [Google Scholar]