Abstract
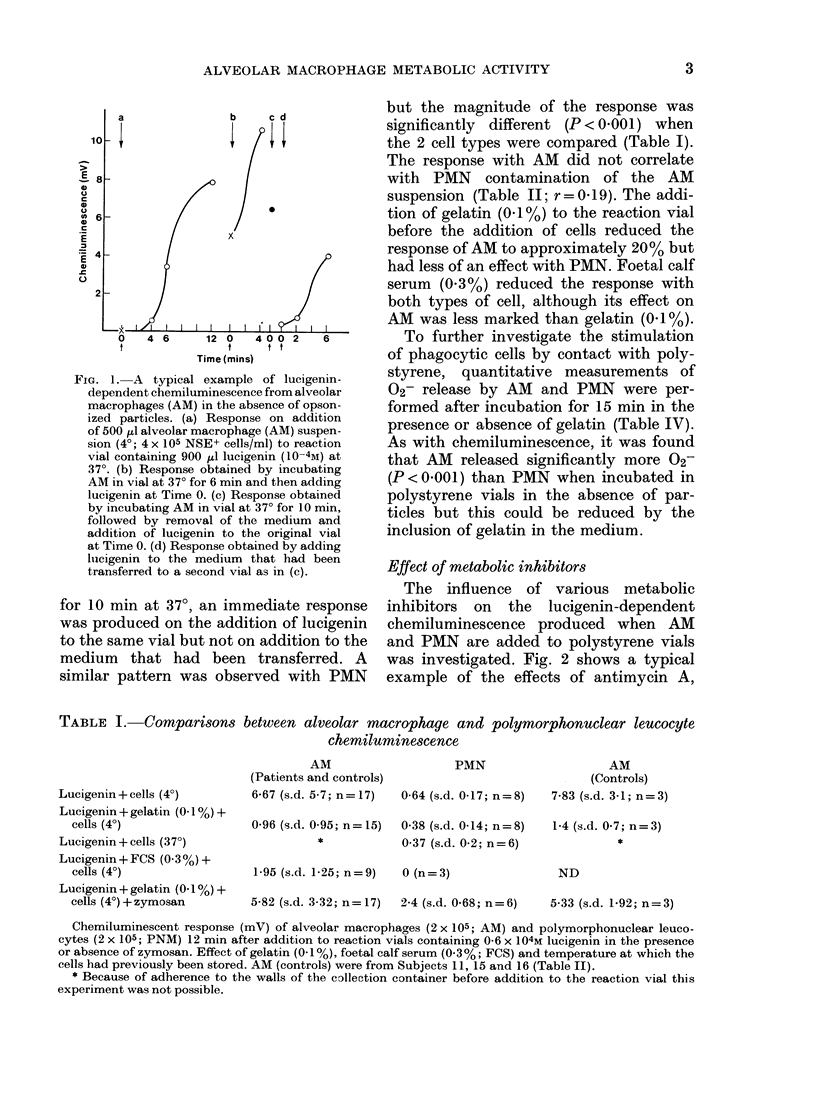
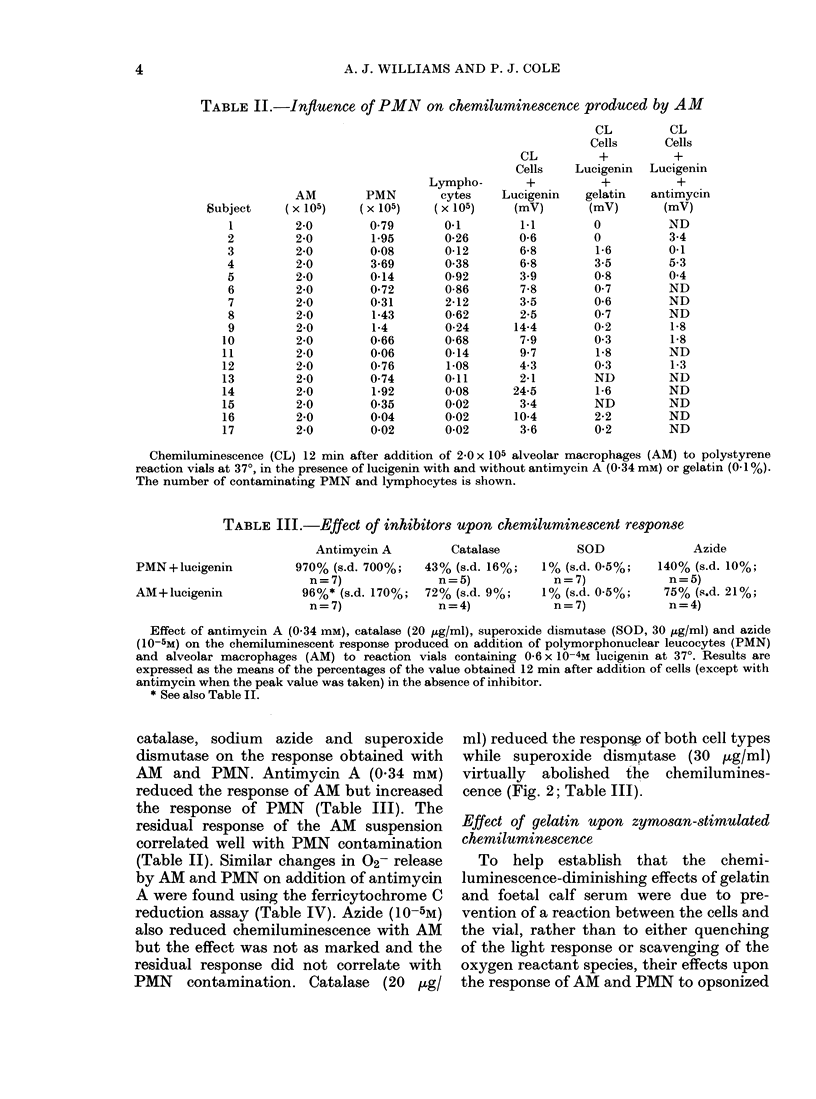
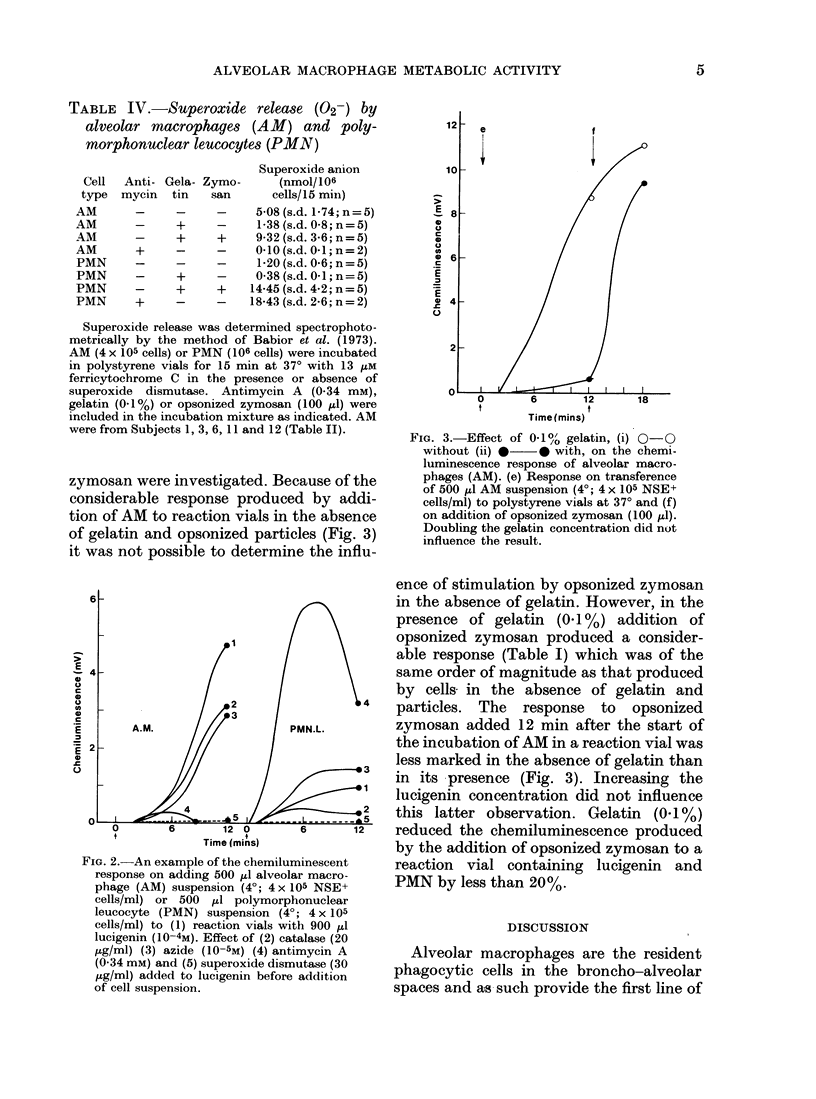
The technique of lucigenin-dependent phagocytic chemiluminescence was used to investigate the stimulation of metabolic activity in human alveolar macrophages on contact with polystyrene. The results were similar to those obtained using a spectrophotometric assay of superoxide release based on ferricytochrome C reduction. There was a marked stimulation of metabolic activity in the alveolar macrophages on incubation at 37 degrees in polystyrene vials which was shown to be due to contact with and adherence to the polystyrene. This could be reduced by the addition of gelatin or foetal calf serum without preventing the ability of the cells to respond to opsonized particles. By using several metabolic inhibitors it was shown that lucigenin-dependent chemiluminescence was associated with the release of superoxide at the time of adherence. The implications of these findings are discussed and it is suggested that the stimulation of alveolar macrophage metabolic activity by contact with polystyrene can contribute to the observed difference between alveolar macrophage and polymorphonuclear leucocyte oxygen consumption in the absence of phagocytosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt L. K., Goetzl E. J. Constituents of human neutrophils that mediate enhanced adherence to surfaces: purification and identification as acidic proteins of the specific granules. J Clin Invest. 1980 Jun;65(6):1372–1381. doi: 10.1172/JCI109801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- Easmon C. S., Cole P. J., Williams A. J., Hastings M. The measurement of opsonic and phagocytic function by Luminol-dependent chemiluminescence. Immunology. 1980 Sep;41(1):67–74. [PMC free article] [PubMed] [Google Scholar]
- Gold L. I., Pearlstein E. Fibronectin-collagen binding and requirement during cellular adhesion. Biochem J. 1980 Feb 15;186(2):551–559. doi: 10.1042/bj1860551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch G. E., Gardner D. E., Menzel D. B. Chemiluminescence of phagocytic cells caused by N-formylmethionyl peptides. J Exp Med. 1978 Jan 1;147(1):182–195. doi: 10.1084/jem.147.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking W. G., Golde D. W. The pulmonary-alveolar macrophage (first of two parts). N Engl J Med. 1979 Sep 13;301(11):580–587. doi: 10.1056/NEJM197909133011104. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., White J. G., Repine J. E. Impairment of human alveolar macrophage oxygen consumption, and superoxide anion production by local anesthetics used in bronchoscopy. Chest. 1979 Feb;75(2 Suppl):243–246. doi: 10.1378/chest.75.2_supplement.243. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. L. The metabolism of leukocytes. Semin Hematol. 1968 Apr;5(2):156–165. [PubMed] [Google Scholar]
- Kiyotaki C., Shimizu A., Watanabe S., Yamamura Y. Superoxide production from human polymorphonuclear leucocytes stimulated with immunoglobulins of different classes and fragments of IgG bound to polystyrene dishes. Immunology. 1978 Oct;35(4):613–618. [PMC free article] [PubMed] [Google Scholar]
- Markert M., Allaz M. J., Frei J. Continuous monitoring of oxygen consumption and superoxide production by particle-stimulated human polymorphonuclear leukocytes. FEBS Lett. 1980 May 5;113(2):225–230. doi: 10.1016/0014-5793(80)80597-7. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Ziprin R. L. Phagocytosis by sheep alveolar macrophages: relationship between opsonin concentration and light emission in the presence of luminol. Infect Immun. 1978 Mar;19(3):889–892. doi: 10.1128/iai.19.3.889-892.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


