Abstract
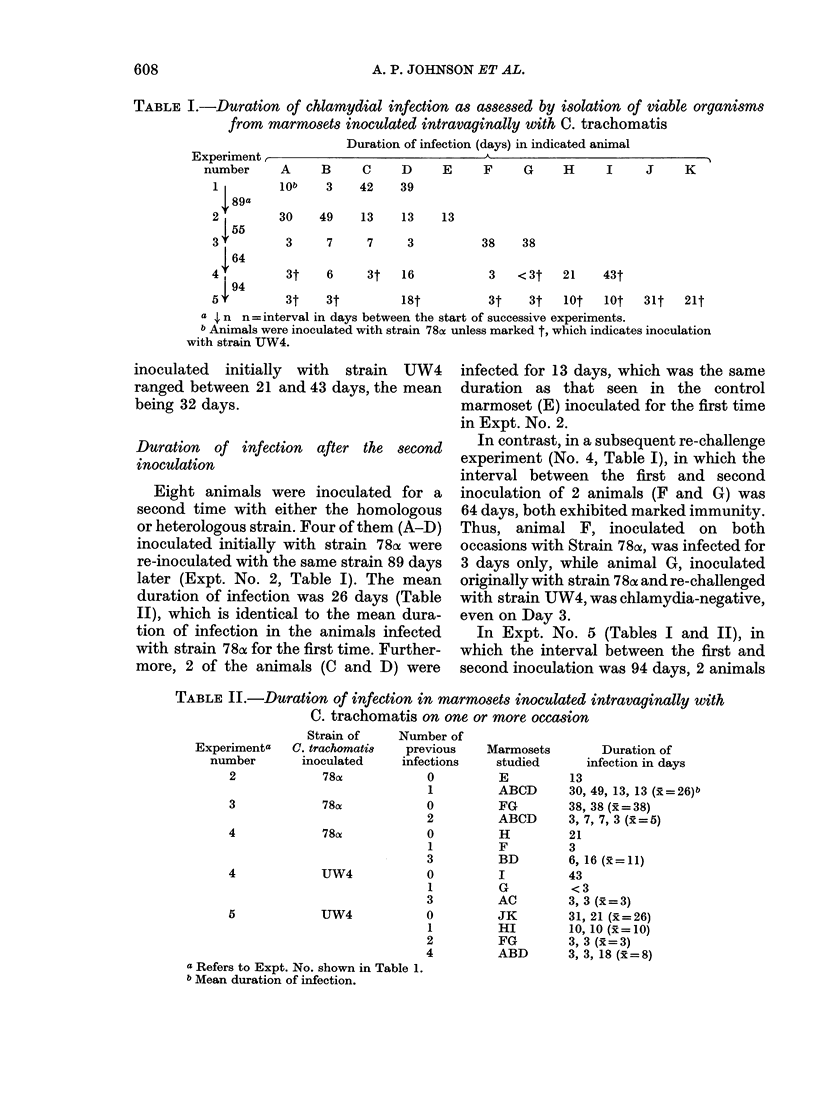
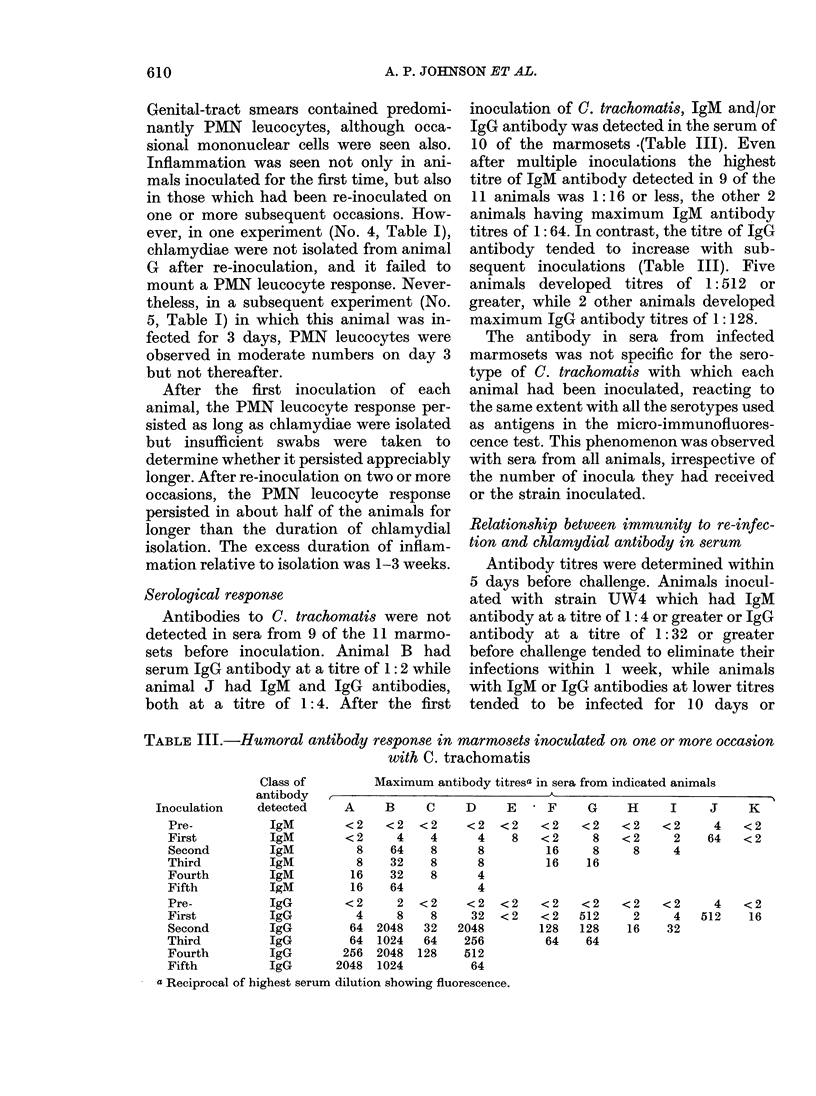
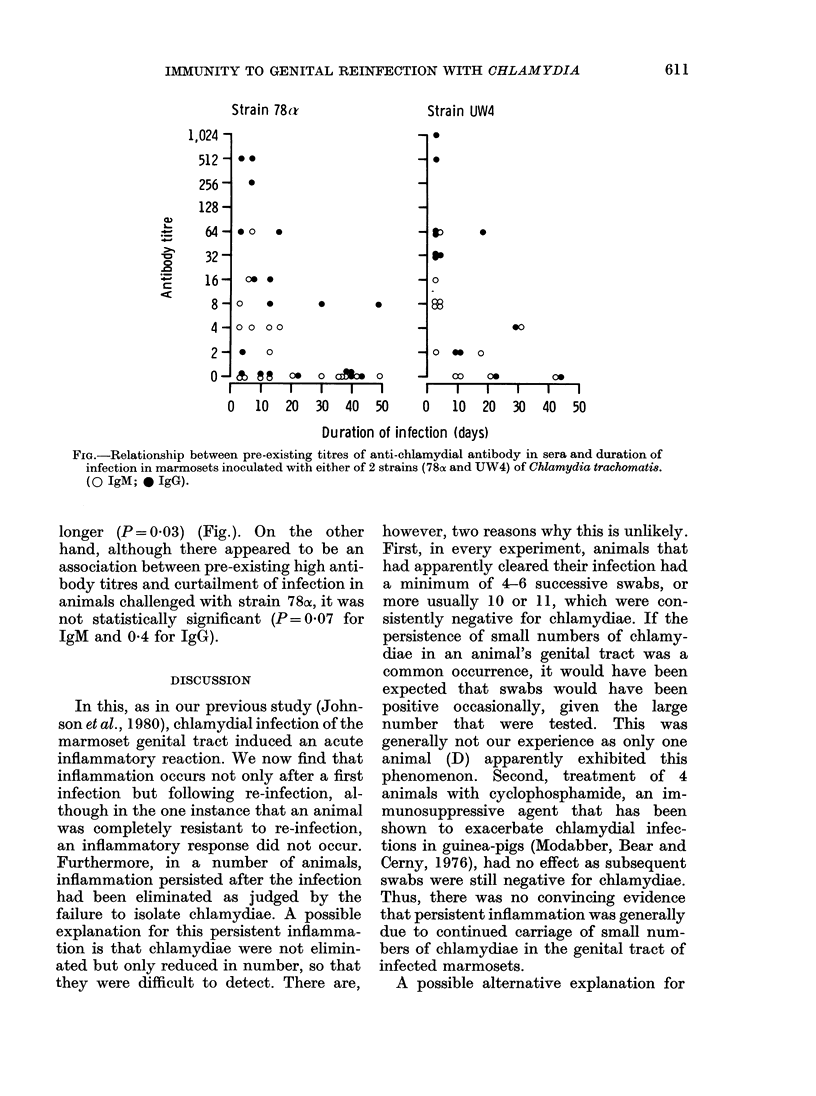
Eleven marmosets inoculated intra-vaginally with either of 2 serotypes (D/E and H) of Chlamydia trachomatis developed a self-limited infection which persisted usually for 10-42 days. Animals re-inoculated on one or more occasions were, however, infected generally for a shorter duration, usually 3-7 days. Curtailed infections were observed after re-inoculation with either the same or a different serotype, indicating that immunity was not serotype specific but cross-protective. IgM and/or IgG chlamydial antibody, measured by micro-immunofluorescence, developed in most of the marmosets on primary infection and was not serotype specific. The antibody titres were boosted on re-infection and there was a correlation between pre-existing high antibody titres and infections of short duration. Chlamydial infection of the genital tract was accompanied by acute inflammation which persisted in about half of the immune animals for up to several weeks despite rapid clearance of the organisms. These features of the experimental infection should help to provide a greater understanding of the immunobiology and pathogenesis of chlamydial genital-tract infections of humans.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Digiacomo R. F., Gale J. L., Wang S. P., Kiviat M. D. Chlamydial infection of the male baboon urethra. Br J Vener Dis. 1975 Oct;51(5):310–313. doi: 10.1136/sti.51.5.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. T., Taylor-Robinson D. Comparison of various McCoy cell treatment procedures used for detection of Chlamydia trachomatis. J Clin Microbiol. 1979 Aug;10(2):198–201. doi: 10.1128/jcm.10.2.198-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr P. M., Taylor-Robinson D., Hetherington C. M. The occurrence of ureaplasmas in marmosets. Lab Anim. 1976 Oct;10(10):393–398. doi: 10.1258/002367776780956944. [DOI] [PubMed] [Google Scholar]
- Gordon F. B., Harper I. A., Quan A. L., Treharne J. D., Dwyer R. S., Garland J. A. Detection of Chlamydia (Bedsonia) in certain infections of man. I. Laboratory procedures: comparison of yolk sac and cell culture for detection and isolation. J Infect Dis. 1969 Oct;120(4):451–462. doi: 10.1093/infdis/120.4.451. [DOI] [PubMed] [Google Scholar]
- Jacobs N. F., Jr, Arum E. S., Kraus S. J. Experimental infection of the chimpanzee urethra and pharynx with Chlamydia trachomatis. Sex Transm Dis. 1978 Oct-Dec;5(4):132–136. doi: 10.1097/00007435-197810000-00002. [DOI] [PubMed] [Google Scholar]
- Johnson A. P., Hetherington C. M., Osborn M. F., Thomas B. J., Taylor-Robinson D. Experimental infection of the marmoset genital tract with Chlamydia trachomatis. Br J Exp Pathol. 1980 Jun;61(3):291–295. [PMC free article] [PubMed] [Google Scholar]
- Modabber F., Bear S. E., Cerny J. The effect of cyclophosphamide on the recovery from a local chlamydial infection. Guinea-pig inclusion conjunctivitis (GPIC). Immunology. 1976 Jun;30(6):929–933. [PMC free article] [PubMed] [Google Scholar]
- Munday P. E., Thomas B. J., Johnson A. P., Altman D. G., Robinson D. T. Clinical and microbiological study of non-gonococcal urethritis with particular reference to non-chlamydial disease. Br J Vener Dis. 1981 Oct;57(5):327–333. doi: 10.1136/sti.57.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripa K. T., Møller B. R., Mårdh P. A., Freundt E. A., Melsen F. Experimental acute salpingitis in grivet monkeys provoked by Chlamydia trachomatis. Acta Pathol Microbiol Scand B. 1979 Feb;87B(1):65–70. doi: 10.1111/j.1699-0463.1979.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Thomas B. J. The rôle of Chlamydia trachomatis in genital-tract and associated diseases. J Clin Pathol. 1980 Mar;33(3):205–233. doi: 10.1136/jcp.33.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. J., Reeve P., Oriel J. D. Simplified serological test for antibodies to Chlamydia trachomatis. J Clin Microbiol. 1976 Jul;4(1):6–10. doi: 10.1128/jcm.4.1.6-10.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Grayston J. T. A simplified method for immunological typing of trachoma-inclusion conjunctivitis-lymphogranuloma venereum organisms. Infect Immun. 1973 Mar;7(3):356–360. doi: 10.1128/iai.7.3.356-360.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


