Abstract
The direct viable count (DVC) method was modified by incorporating radiolabeled substrates in microautoradiographic analyses to assess bacterial survival in controlled laboratory microcosms. The DVC method, which permits enumeration of culturable and nonculturable cells, discriminates those cells that are responsive to added nutrients but in which division is inhibited by the addition of nalidixic acid. The resulting elongated cells represent all viable cells; this includes those that are culturable on routine media and those that are not. Escherichia coli and Salmonella enteritidis were employed in the microcosm studies, and radiolabeled substrates included [methyl-3H]thymidine or [U-14C]glutamic acid. Samples taken at selected intervals during the survival experiments were examined by epifluorescence microscopy to enumerate cells by the DVC and acridine orange direct count methods, as well as by culture methods. Good correlation was obtained for cell-associated metabolic activity, measured by microautoradiography and substrate responsiveness (by the DVC method) at various stages of survival. Of the cells responsive to nutrients by the DVC method, ca. 90% were metabolically active by the microautoradiographic method. No significant difference was observed between DVC enumerations with or without added radiolabeled substrate.
Full text
PDF
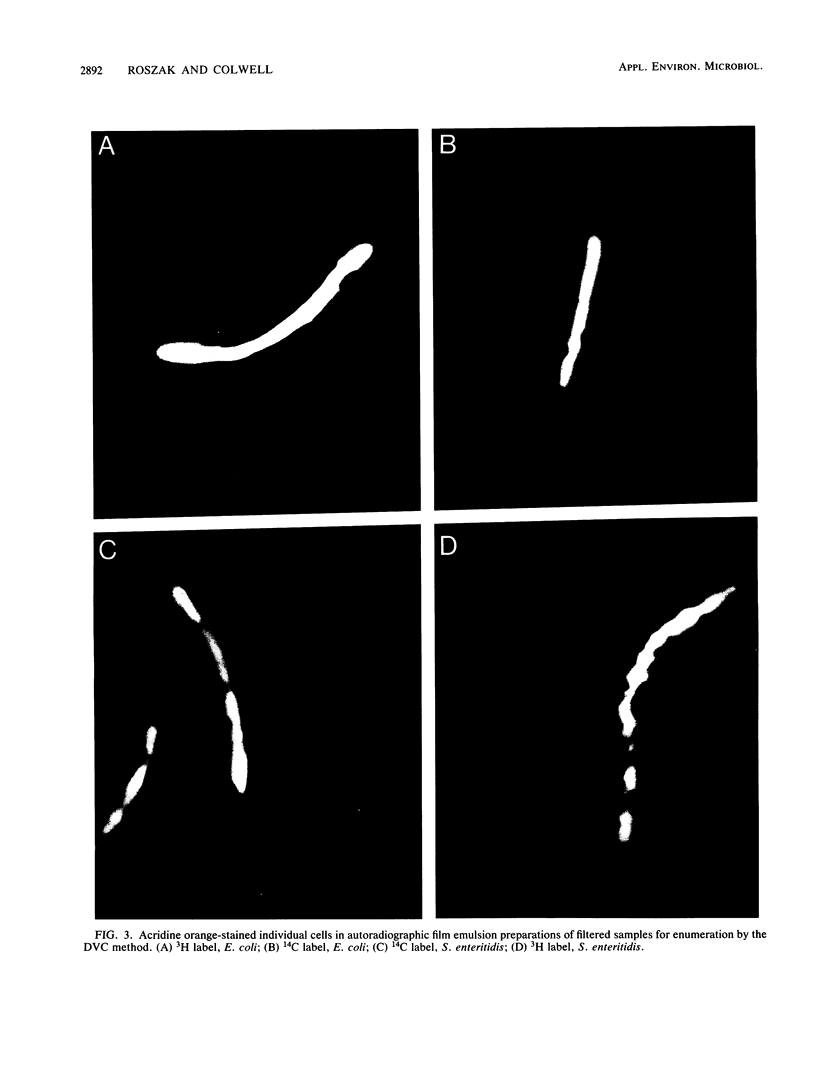

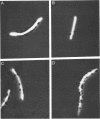
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. D., Brock M. L. Autoradiography as a tool in microbial ecology. Nature. 1966 Feb 12;209(5024):734–736. doi: 10.1038/209734a0. [DOI] [PubMed] [Google Scholar]
- Deitz W. H., Cook T. M., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. 3. Conditions required for lethality. J Bacteriol. 1966 Feb;91(2):768–773. doi: 10.1128/jb.91.2.768-773.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J., Saukkonen J. J. Membrane permeability changes associated with DNA gyrase inhibitors in Escherichia coli. Antimicrob Agents Chemother. 1985 Aug;28(2):200–206. doi: 10.1128/aac.28.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliermans C. B., Schmidt E. L. Autoradiography and immunofluorescence combined for autecological study of single cell activity with Nitrobacter as a model system. Appl Microbiol. 1975 Oct;30(4):676–684. doi: 10.1128/am.30.4.676-684.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor G. J., Deering R. A. Effect of nalidixic acid and hydroxyurea on division ability of Escherichia coli fil+ and lon- strains. J Bacteriol. 1968 Feb;95(2):520–530. doi: 10.1128/jb.95.2.520-530.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Kurath G., Morita R. Y. Starvation-Survival Physiological Studies of a Marine Pseudomonas sp. Appl Environ Microbiol. 1983 Apr;45(4):1206–1211. doi: 10.1128/aem.45.4.1206-1211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Meyer-Reil L. A. Autoradiography and epifluorescence microscopy combined for the determination of number and spectrum of actively metabolizing bacteria in natural water. Appl Environ Microbiol. 1978 Sep;36(3):506–512. doi: 10.1128/aem.36.3.506-512.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Tabor P. S., Neihof R. A. Direct determination of activities for microorganisms of chesapeake bay populations. Appl Environ Microbiol. 1984 Nov;48(5):1012–1019. doi: 10.1128/aem.48.5.1012-1019.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor P. S., Neihof R. A. Improved microautoradiographic method to determine individual microorganisms active in substrate uptake in natural waters. Appl Environ Microbiol. 1982 Oct;44(4):945–953. doi: 10.1128/aem.44.4.945-953.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]