Abstract
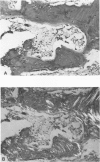
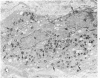
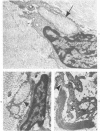
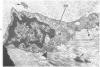
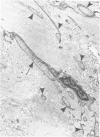
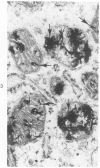
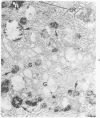
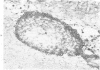
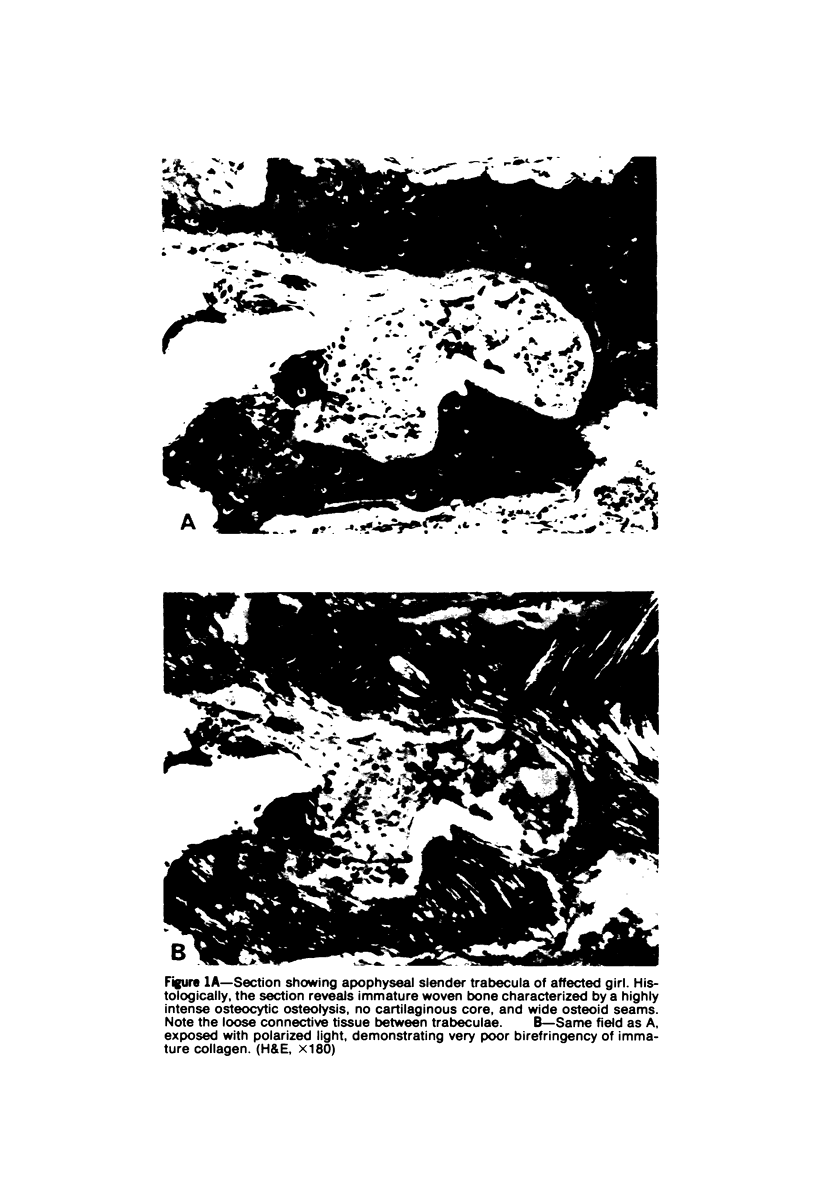
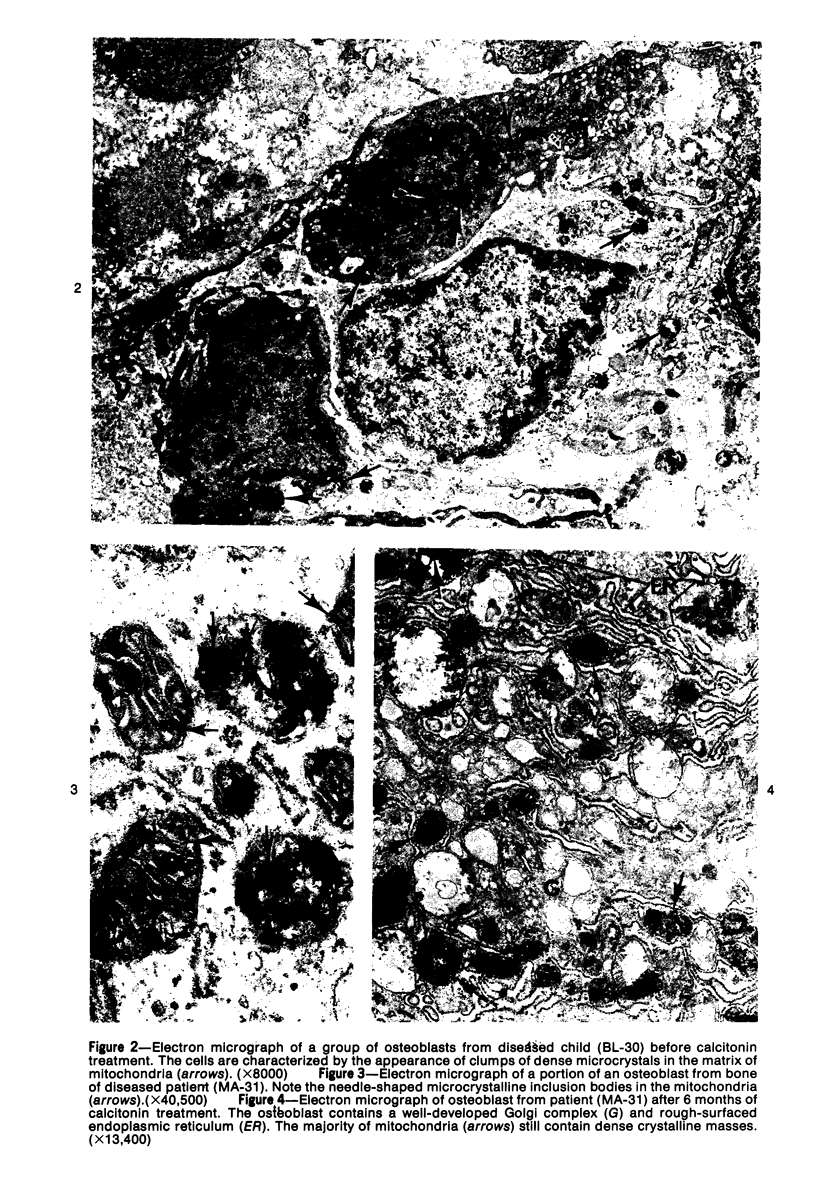
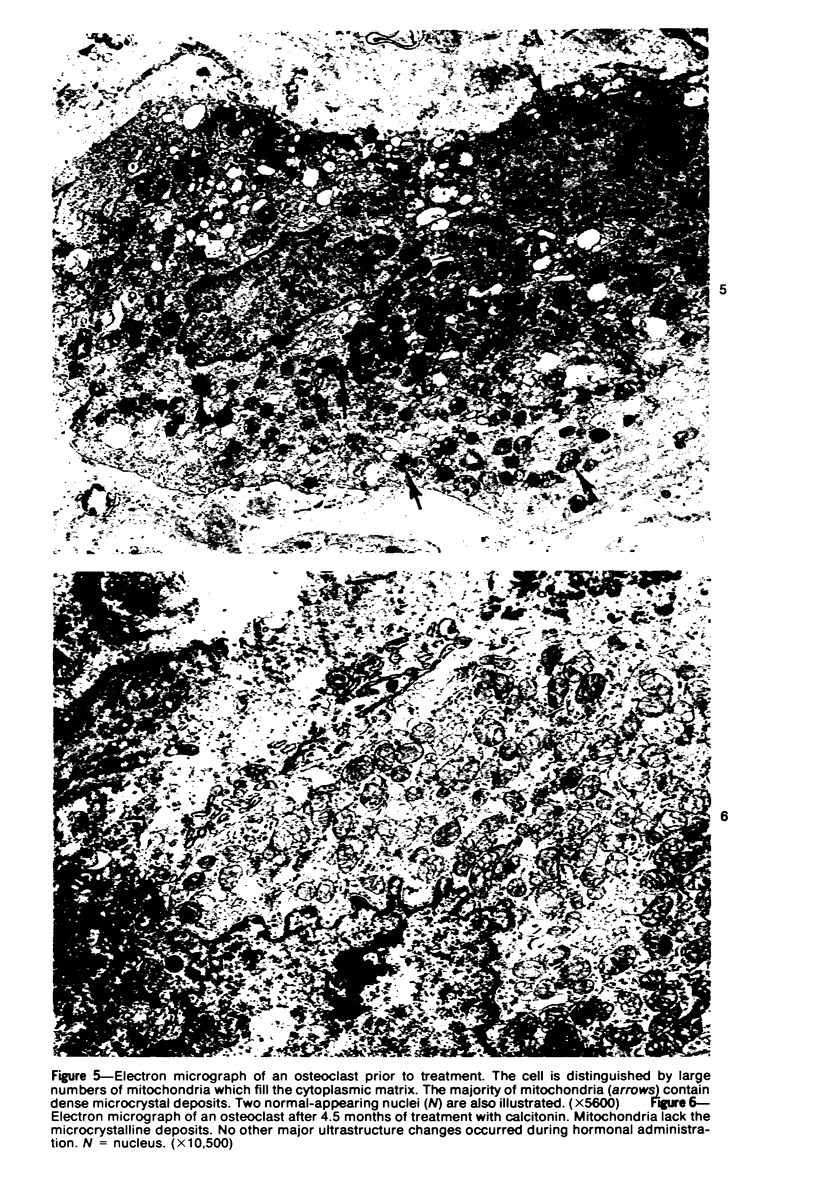
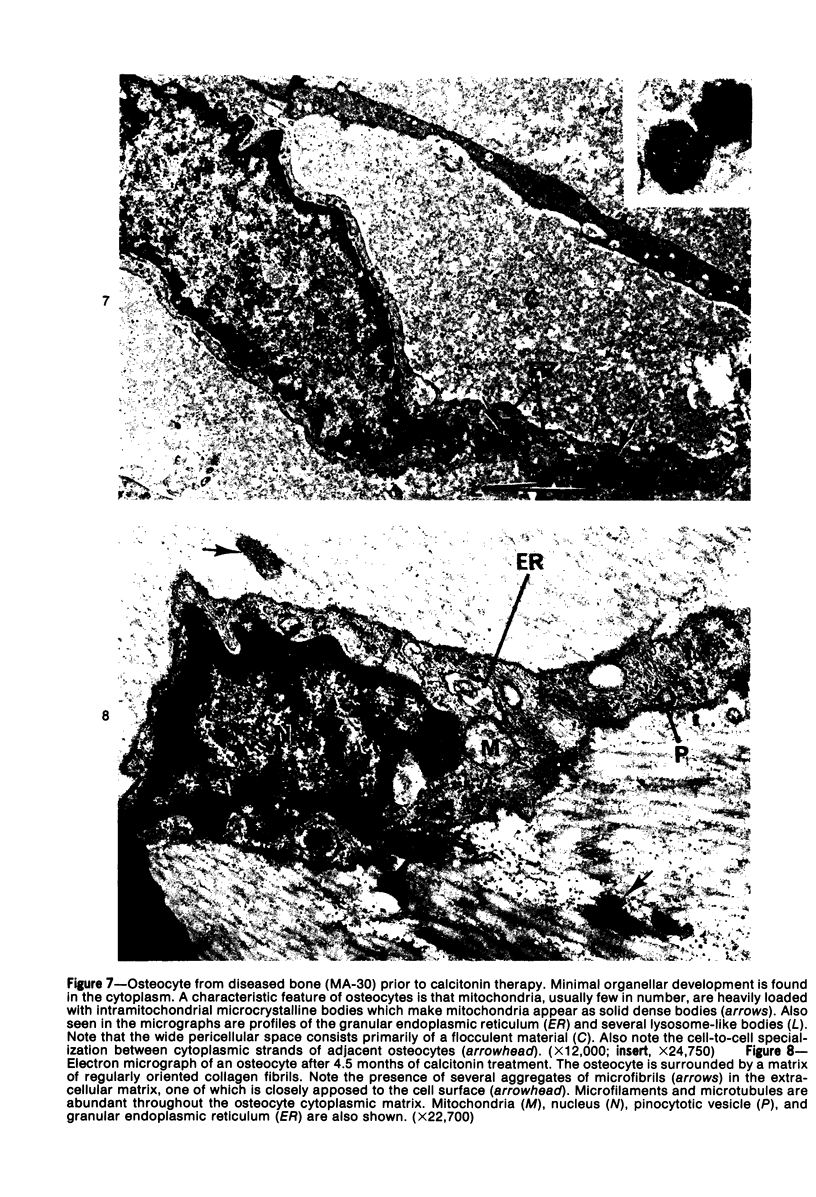
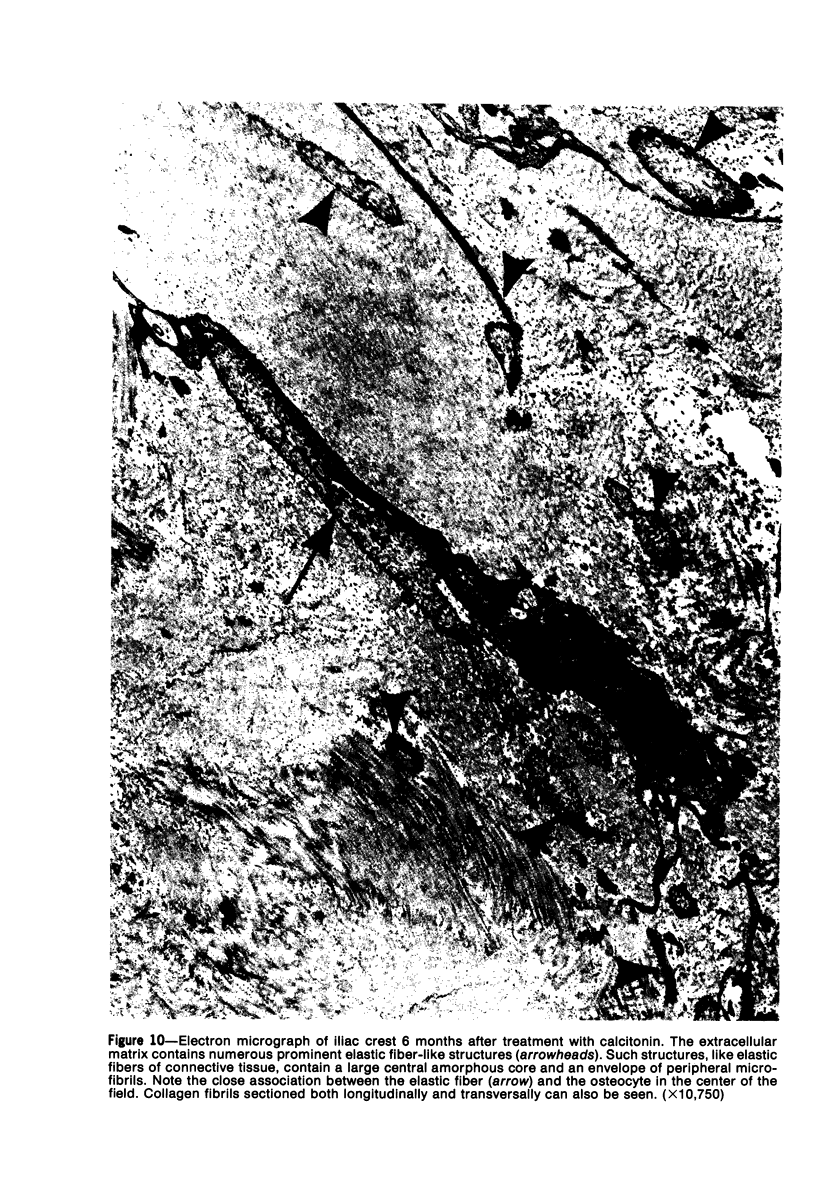
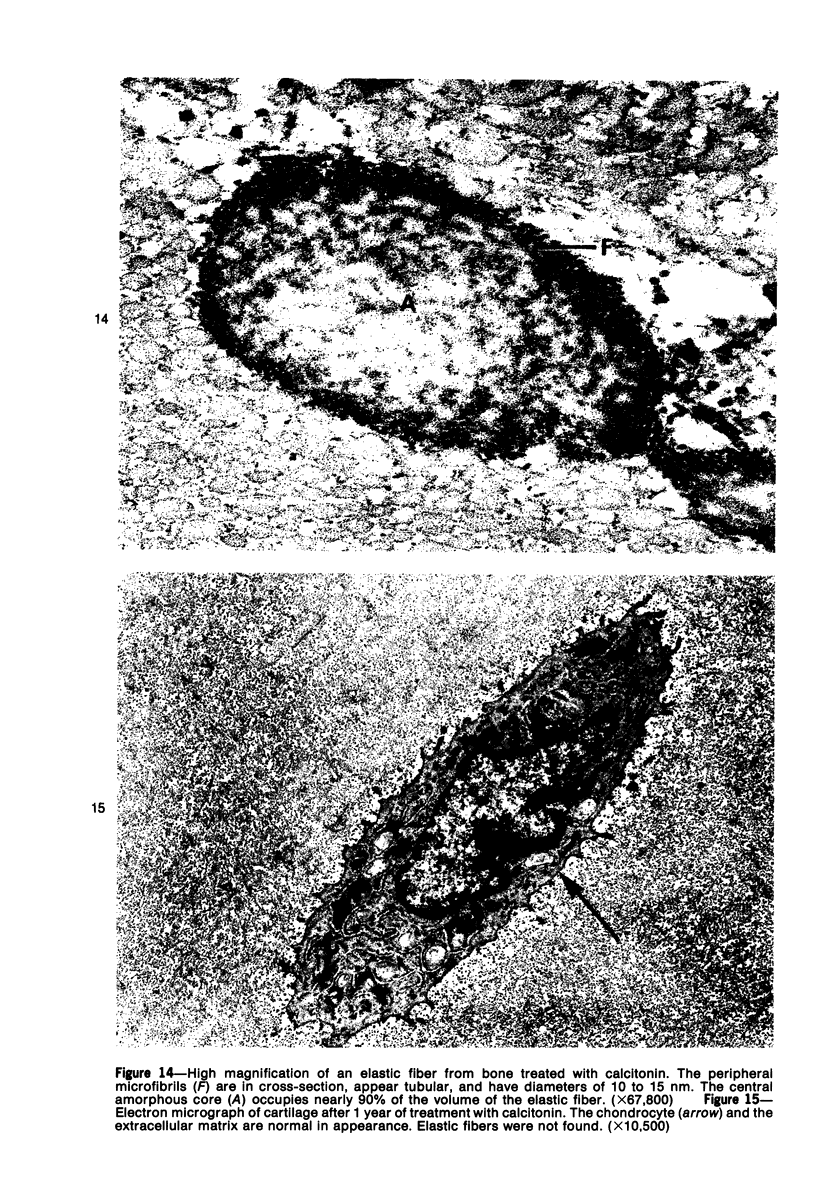
Familial bone dysplasia with hyperphosphatasemia is characterized by excessive bone resorption early in life with resulting severe skeletal deformity. The disease can be ameliorated by treatment with human calcitonin. We have the studied the ultrastructure of bone from diseased patients before treatment and at intervals during 1 year of treatment with calcitonin. Pretreatment osteoblasts, osteoclasts, and osteocytes exhibited mitochondria which contained vast amounts of dense microcrystal deposits. Osteocytes were also distinguished by minimal organellar development. Osteoclasts were rare. Calcitonin treatment included a progressive development of a more normal bone structure. Intramitochondrial crystal deposits were absent in mitochondria of osteocytes and osteoclasts but were still present in mitochondria of osteoblasts. Surprisingly, the developing bony matrix during calcitonin treatment exhibited large numbers of elastic fibers. These appeared to develop normally in alignment with the surface membrane of osteocytes. Calcitonin treatment caused a proliferation of osteocyte organellar development. It is concluded that familial bone dysplasia is primarily a disease of osteocytes and that osteocytic activity is influenced by calcitonin.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKWIN H., EIGER M. S. Fragile bones and macrocranium. J Pediatr. 1956 Nov;49(5):558–564. doi: 10.1016/s0022-3476(56)80143-1. [DOI] [PubMed] [Google Scholar]
- Baud C. A. Submicroscopic structure and functional aspects of the osteocyte. Clin Orthop Relat Res. 1968 Jan-Feb;56:227–236. [PubMed] [Google Scholar]
- Baylink D. J., Wergedal J. E. Bone formation by osteocytes. Am J Physiol. 1971 Sep;221(3):669–678. doi: 10.1152/ajplegacy.1971.221.3.669. [DOI] [PubMed] [Google Scholar]
- Bloom S., Cancilla P. A. Myocytolysis and mitochondrial calcification in rat myocardium after low doses of isoproterenol. Am J Pathol. 1969 Mar;54(3):373–391. [PMC free article] [PubMed] [Google Scholar]
- Bonilla E., Schotland D. L., DiMauro S., Aldover B. Electron cytochemistry of crystalline inclusions in human skeletal muscle mitochondria. J Ultrastruct Res. 1975 Jun;51(3):404–408. doi: 10.1016/s0022-5320(75)80103-1. [DOI] [PubMed] [Google Scholar]
- Bonucci E., Gherardi G. Osteocyte ultrastructure in renal osteodystrophy. Virchows Arch A Pathol Anat Histol. 1977 Apr 6;373(3):213–231. doi: 10.1007/BF00432238. [DOI] [PubMed] [Google Scholar]
- Bélanger L. F. Osteocytic osteolysis. Calcif Tissue Res. 1969 Aug 11;4(1):1–12. doi: 10.1007/BF02279101. [DOI] [PubMed] [Google Scholar]
- Cavallero C., Spagnoli L. G., Di Tondo U. Early mitochondrial calcifications in the rabbit aorta after adrenaline. Virchows Arch A Pathol Anat Histol. 1974;362(1):23–39. doi: 10.1007/BF00433772. [DOI] [PubMed] [Google Scholar]
- D AGOSTINO A. N. AN ELECTRON MICROSCOPIC STUDY OF CARDIAC NECROSIS PRODUCED BY 9 ALPHA-FLUOROCORTISOL AND SODIUM PHOSPHATE. Am J Pathol. 1964 Oct;45:633–644. [PMC free article] [PubMed] [Google Scholar]
- D'Agostino A. N., Chiga M. Morphologic changes in cardiac and skeletal muscle induced by corticosteroids. Ann N Y Acad Sci. 1966 Sep 9;138(1):73–81. doi: 10.1111/j.1749-6632.1966.tb41156.x. [DOI] [PubMed] [Google Scholar]
- Eyring E. J., Eisenberg E. Congenital hyperphosphatasia. A clinical, pathological, and biochemical study of two cases. J Bone Joint Surg Am. 1968 Sep;50(6):1099–1117. [PubMed] [Google Scholar]
- Greenlee T. K., Jr, Ross R., Hartman J. L. The fine structure of elastic fibers. J Cell Biol. 1966 Jul;30(1):59–71. doi: 10.1083/jcb.30.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee T. K., Jr, Ross R. The development of the rat flexor digital tendon, a fine structure study. J Ultrastruct Res. 1967 May;18(3):354–376. doi: 10.1016/s0022-5320(67)80124-2. [DOI] [PubMed] [Google Scholar]
- Jande S. S., Bélanger L. F. The life cycle of the osteocyte. Clin Orthop Relat Res. 1973 Jul-Aug;(94):281–305. doi: 10.1097/00003086-197307000-00035. [DOI] [PubMed] [Google Scholar]
- Jande S. S. Fine structural study of osteocytes and their surrounding bone matrix with respect to their age in young chicks. J Ultrastruct Res. 1971 Nov;37(3):279–300. doi: 10.1016/s0022-5320(71)80125-9. [DOI] [PubMed] [Google Scholar]
- Krook L., Bélanger L. F., Henrikson P. A., Lutwak L., Sheffy B. E. Bone flow. Rev Can Biol. 1970 Jun;29(2):157–167. [PubMed] [Google Scholar]
- Lin J. J. Intramitochondrial calcification in infant myocardium. Occurrence in a case of coarctation of aorta. Arch Pathol. 1972 Oct;94(4):366–369. [PubMed] [Google Scholar]
- Luk S. C., Nopajaroonsri C., Simon G. T. The ultrastructure of cortical bone in young adult rabbits. J Ultrastruct Res. 1974 Feb;46(2):184–205. doi: 10.1016/s0022-5320(74)80055-9. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr Lack of effect of thyrocalcitonin on formation of bone matrix in mice and rats. Horm Metab Res. 1972 Jul;4(4):296–300. doi: 10.1055/s-0028-1094071. [DOI] [PubMed] [Google Scholar]
- Mills B. G., Singer F. R. Nuclear inclusions in Paget's disease of bone. Science. 1976 Oct 8;194(4261):201–202. doi: 10.1126/science.959849. [DOI] [PubMed] [Google Scholar]
- Ross R., Bornstein P. The elastic fiber. I. The separation and partial characterization of its macromolecular components. J Cell Biol. 1969 Feb;40(2):366–381. doi: 10.1083/jcb.40.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The elastic fiber. J Histochem Cytochem. 1973 Mar;21(3):199–208. doi: 10.1177/21.3.199. [DOI] [PubMed] [Google Scholar]
- Stemmermann G. N. An histologic and histochemical study of familial osteoectasia. (Chronic idiopathic hyperphosphatasia). Am J Pathol. 1966 Apr;48(4):641–651. [PMC free article] [PubMed] [Google Scholar]
- Vittali P. H. Osteocyte activity. Clin Orthop Relat Res. 1968 Jan-Feb;56:213–226. [PubMed] [Google Scholar]
- Wassermann F., Yaeger J. A. The matrices of mineralizable tissues. Int Dent J. 1969 Jun;19(2):308–343. [PubMed] [Google Scholar]
- Whalen J. P., Horwith M., Krook L., MacIntyre I., Mena E., Viteri F., Torun B., Nunez E. A. Calcitonin treatment in hereditary bone dysplasia with hyperphosphatasemia: a radiographic and histologic study of bone. AJR Am J Roentgenol. 1977 Jul;129(1):29–35. doi: 10.2214/ajr.129.1.29. [DOI] [PubMed] [Google Scholar]
- Whalen J. P., O'Donohue N., Krook L., Nunez E. A. Pathogenesis of abnormal remodeling of bones: effects of yellow phosphorus in the growing rat. Anat Rec. 1973 Sep;177(1):15–22. doi: 10.1002/ar.1091770103. [DOI] [PubMed] [Google Scholar]