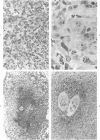
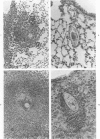
Abstract
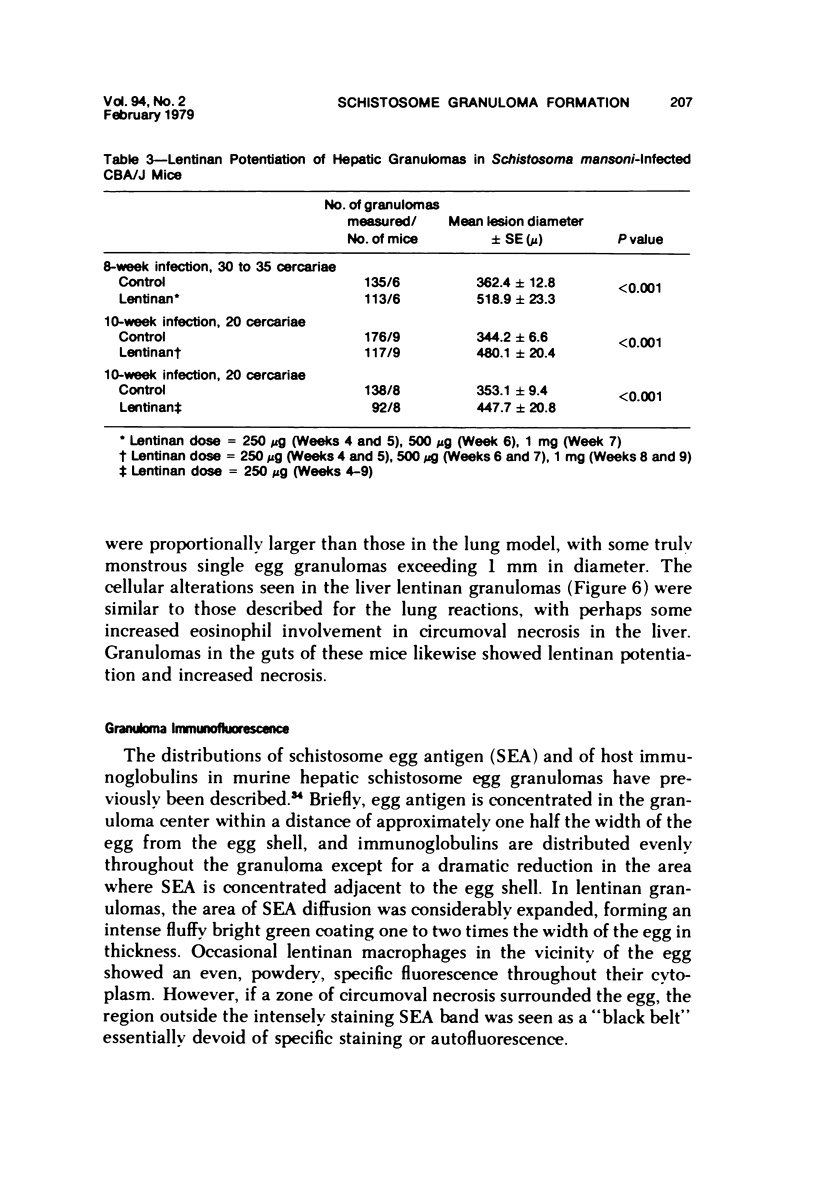
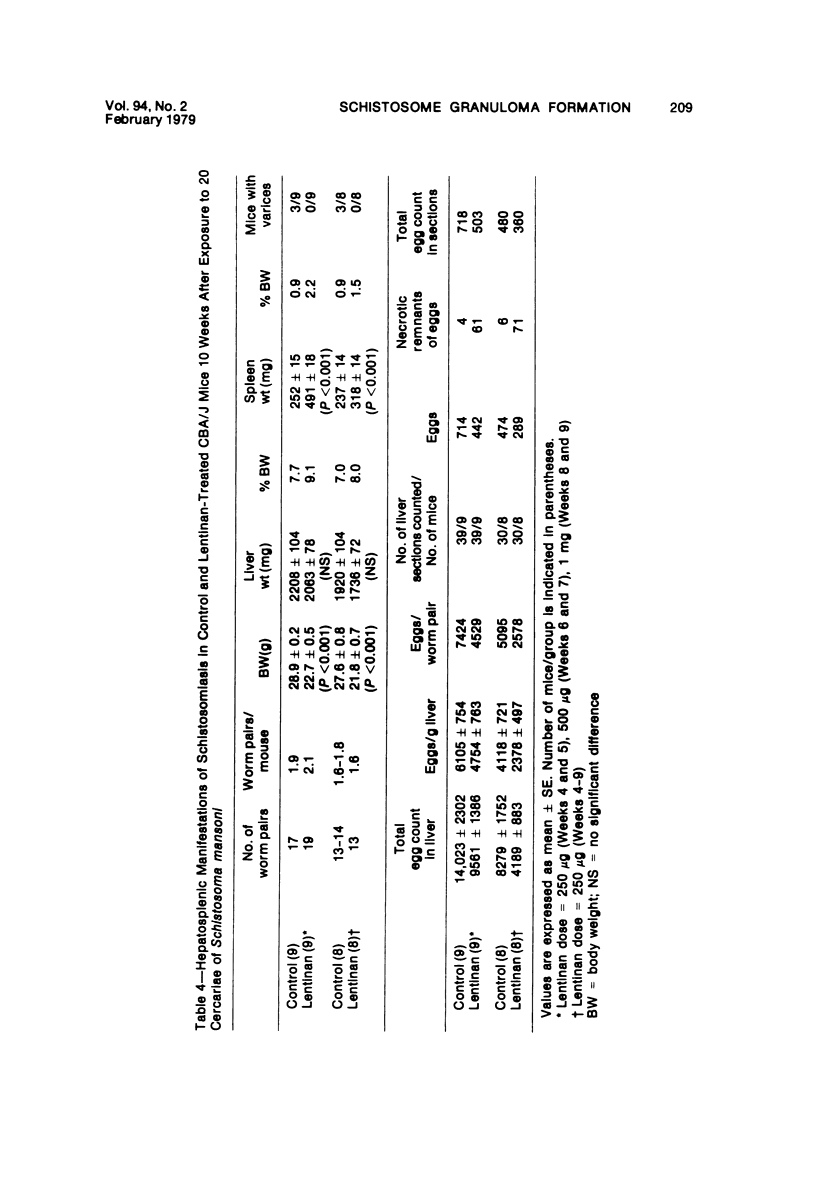
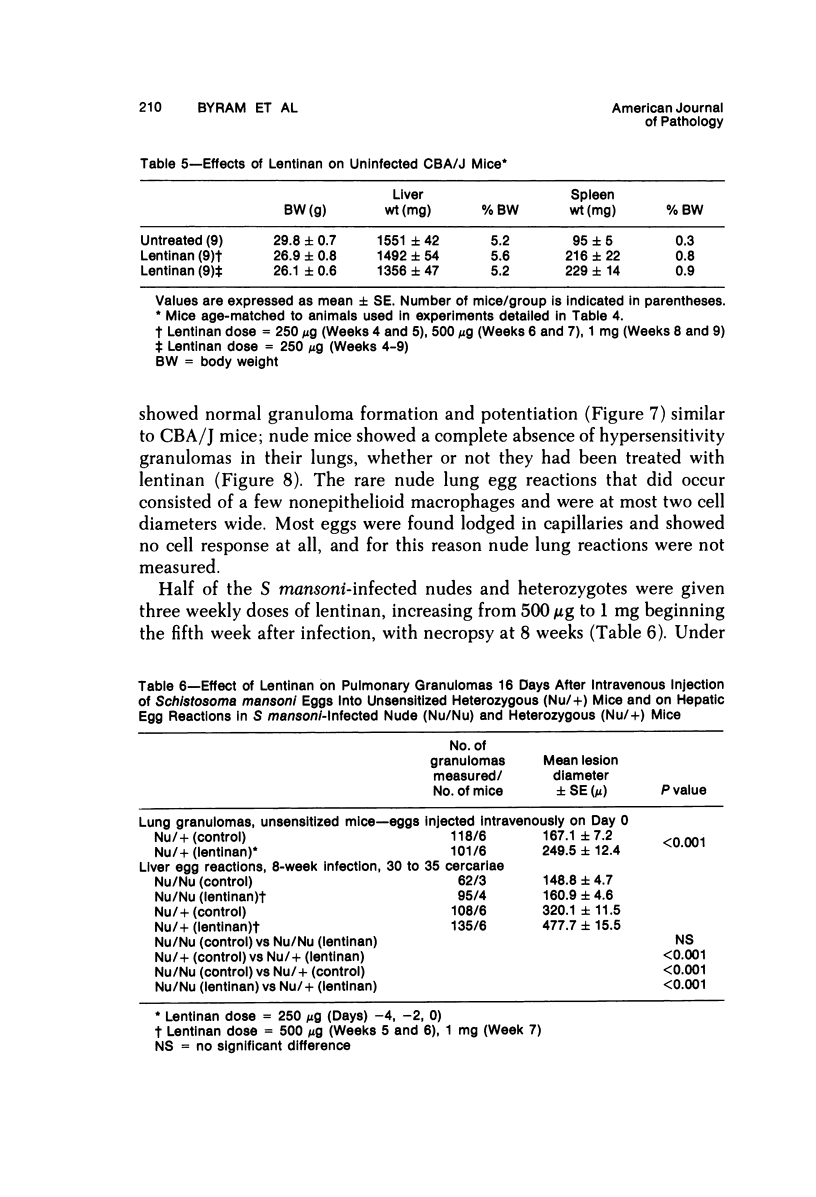
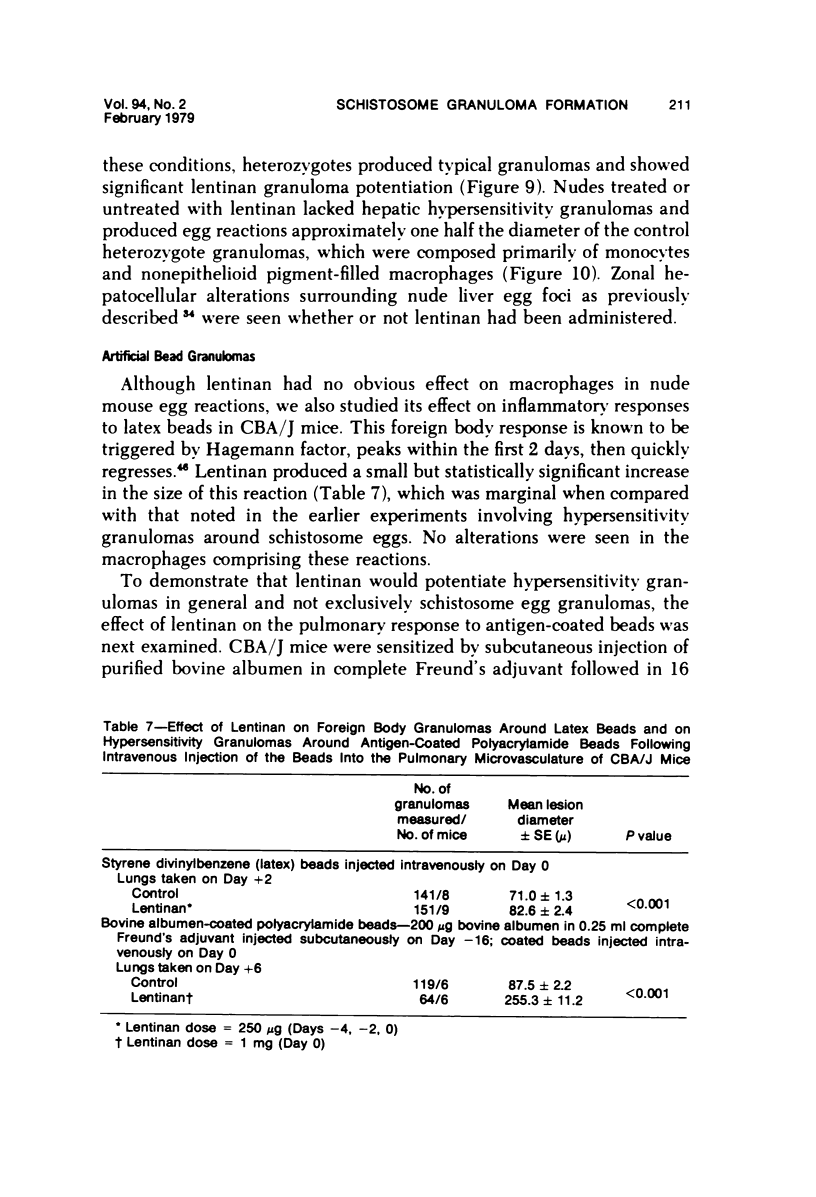
Lentinan is a fungal polysaccharide which acts as a T-cell adjuvant. When this glucan was administered to thymus-intact mice by intraperitoneal injection, conspicuously enlarged lung granulomas formed in response to either Schistosoma mansoni or S japonicum eggs or to antigen-coated polyacrylamide beads. Liver granulomas in cercaria-induced S mansoni infection were augmented up to eight-fold in volume. By contrast, nude mice showed a complete absence of hypersensitivity granulomas, regardless of whether they received lentinan. Lentinan-potentiated granulomas show a distinctive histopathologic picture characterized by abundant, large, pale-staining macrophages; reduced and redistributed eosinophil populations; and frequent, extensive central necrosis, uncommon in unpotentiated schistosome foci. They also differ in their distributions of egg antigen and of host immunoglobulins. Optimal lentinan effects followed a single 1-mg dose when given to sensitized mice on the day of intravenous challenge with S mansoni eggs rather than at the time of intraperitoneal sensitization or following challenge. This adjuvant appears to act on effector T cells or on macrophages interacting with T cells; its effect on macrophages in a latex bead foreign body granuloma was minimal. A number of other lentinan-associated systemic effects on parasite and host were noted and described, including reduced female schistosome egg output.
Full text
PDF












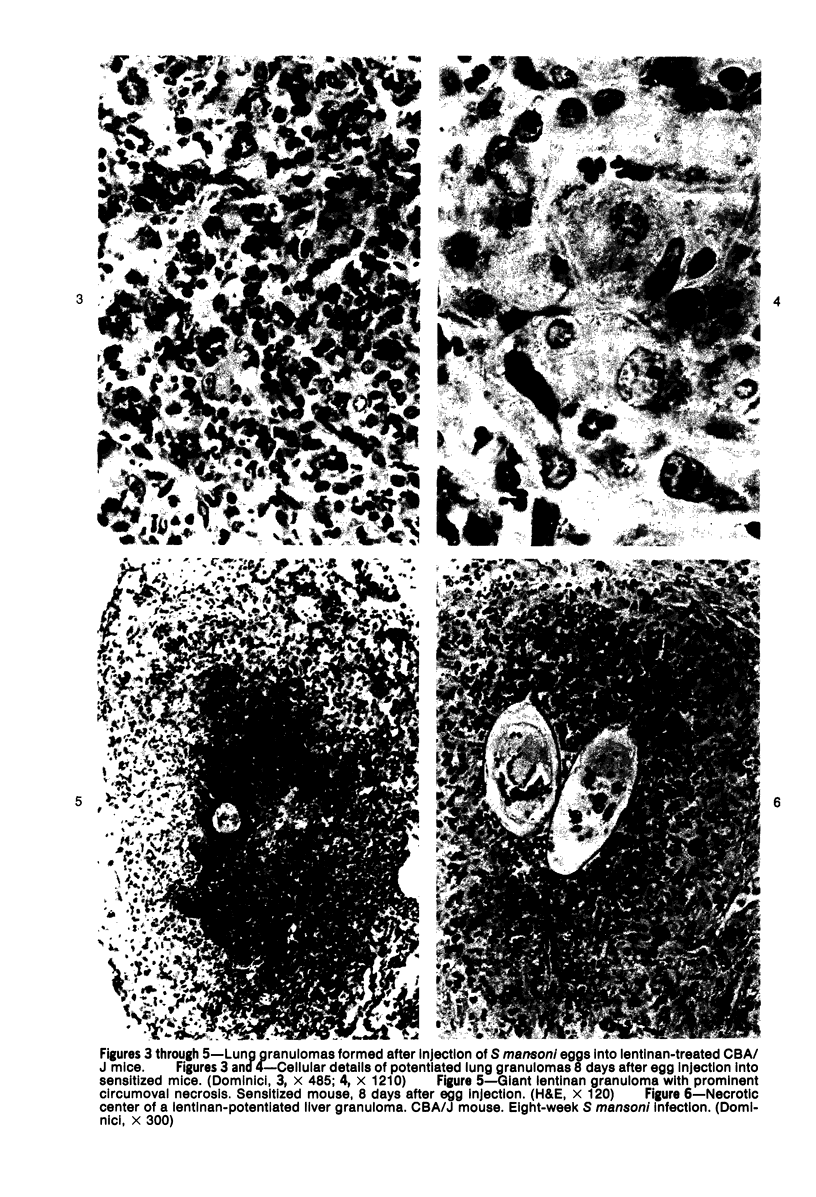
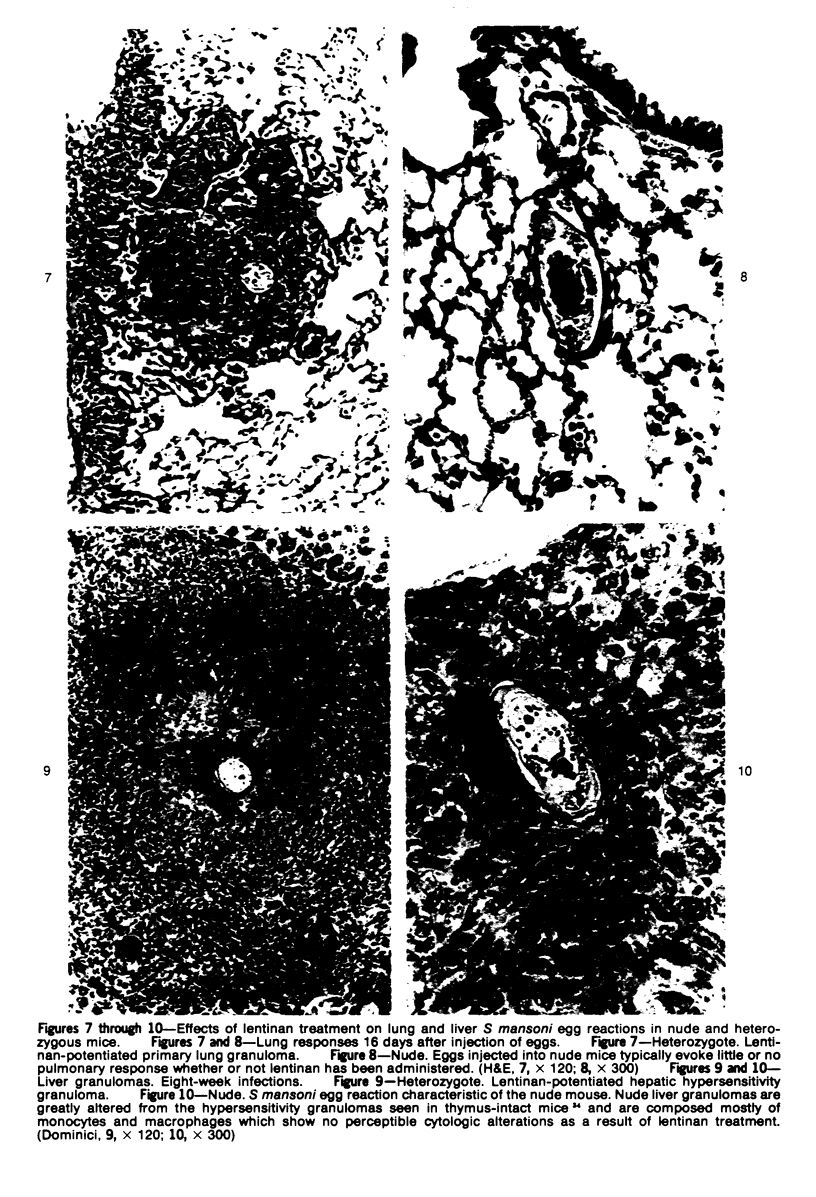
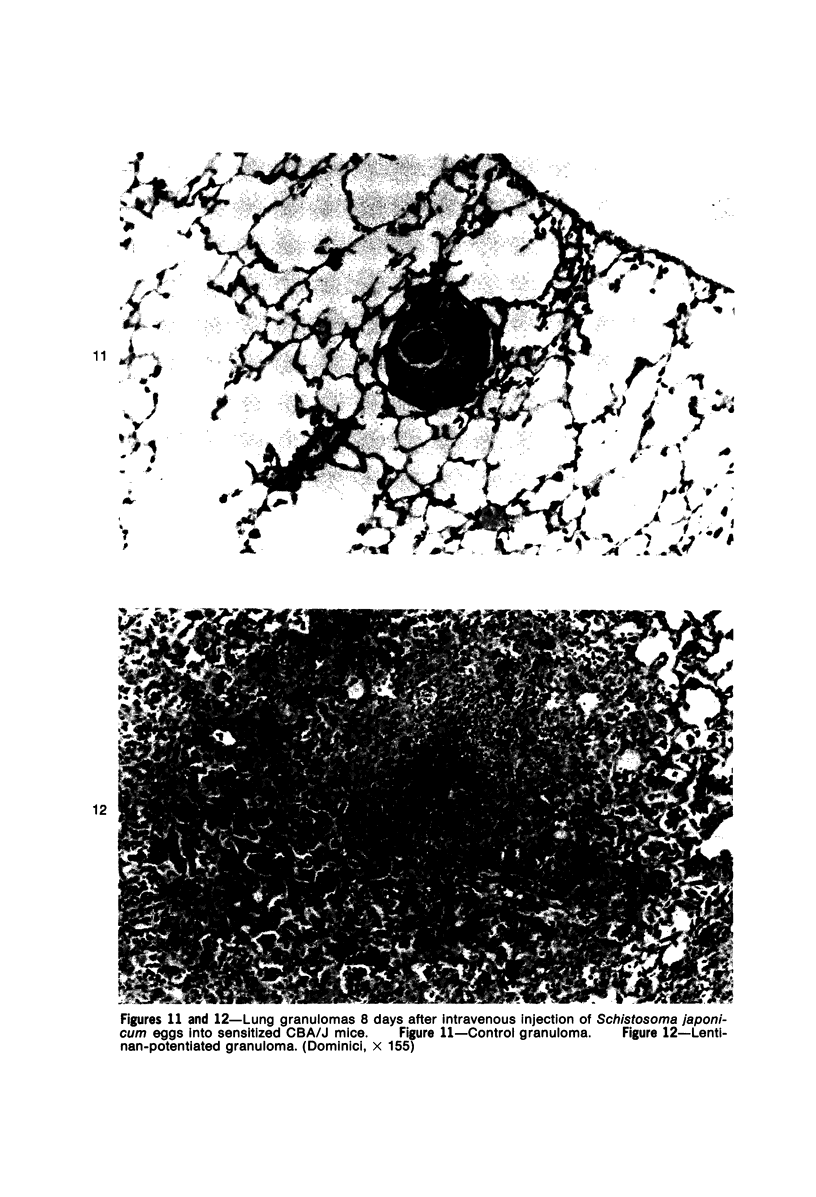
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Wahab M. F., Powers K. G., Mahmoud S. S., Good W. C. Suppression of schistosome granuloma formation by malaria in mice. Am J Trop Med Hyg. 1974 Sep;23(5):915–918. doi: 10.4269/ajtmh.1974.23.915. [DOI] [PubMed] [Google Scholar]
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Berenberg J. L., Ward P. A. Chemotactic factor inactivator in normal human serum. J Clin Invest. 1973 May;52(5):1200–1206. doi: 10.1172/JCI107287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomford R., Moreno C. Mechanism of the anti-tumour effect of glucans and fructosans: a comparison with C. parvum. Br J Cancer. 1977 Jul;36(1):41–48. doi: 10.1038/bjc.1977.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan R. D., Fine D. P., Colley D. G. Schistosoma mansoni infection in mice depleted of thymus-dependent lymphocytes. II. Pathology and altered pathogenesis. Am J Pathol. 1973 May;71(2):207–218. [PMC free article] [PubMed] [Google Scholar]
- Byram J. E., von Lichtenberg F. Altered schistosome granuloma formation in nude mice. Am J Trop Med Hyg. 1977 Sep;26(5 Pt 1):944–956. doi: 10.4269/ajtmh.1977.26.944. [DOI] [PubMed] [Google Scholar]
- COKER C. M., LICHTENBERG F. A revised method for isolation of Schistosoma mansoni eggs for biological experimentation. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):780–782. doi: 10.3181/00379727-92-22612. [DOI] [PubMed] [Google Scholar]
- Cheever A. W. Conditions affecting the accuracy of potassium hydroxide digestion techniques for counting Schistosoma mansoni eggs in tissues. Bull World Health Organ. 1968;39(2):328–331. [PMC free article] [PubMed] [Google Scholar]
- Chihara G., Hamuro J., Maeda Y., Arai Y., Fukuoka F. Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing. (an edible mushroom). Cancer Res. 1970 Nov;30(11):2776–2781. [PubMed] [Google Scholar]
- Colley D. G. Adoptive suppression of granuloma formation. J Exp Med. 1976 Mar 1;143(3):696–700. doi: 10.1084/jem.143.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley D. G., Lewis F. A., Goodgame R. W. Immune responses during human schistosomiasis mansoni. IV. Induction of suppressor cell activity by schistosome antigen preparations and concanavalin A. J Immunol. 1978 Apr;120(4):1225–1232. [PubMed] [Google Scholar]
- Coulis P. A., Lewert R. M., Fitch F. W. Splenic suppressor cells and cell-mediated cytotoxicity in murine schistosomiasis. J Immunol. 1978 Mar;120(3):1074–1076. [PubMed] [Google Scholar]
- Dennert G., Tucker D. Antitumor polysaccharide lentinan. A T cell adjuvant. J Natl Cancer Inst. 1973 Nov;51(5):1727–1729. doi: 10.1093/jnci/51.5.1727. [DOI] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. The inhibition of granuloma formation around Schistosoma mansoni eggs. 3. Heterologous antilymphocyte serum. Am J Pathol. 1968 Mar;52(3):613–631. [PMC free article] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. The inhibition of granuloma formation around Schistosoma mansoni eggs. II. Thymectomy. Am J Pathol. 1967 Nov;51(5):757–767. [PMC free article] [PubMed] [Google Scholar]
- Dresser D. W., Phillips J. M. The orientation of the adjuvant activities of Salmonella typhosa lipopolysaccharide and lentinan. Immunology. 1974 Nov;27(5):895–902. [PMC free article] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Effects of concanavalin A on mouse peritoneal macrophages. I. Stimulation of endocytic activity and inhibition of phago-lysosome formation. J Exp Med. 1974 Nov 1;140(5):1364–1386. doi: 10.1084/jem.140.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo B., Imaeda T. Cellular response to Freund's adjuvant in the rabbit lung. An electron microscope study. Lab Invest. 1966 Nov;15(11):1659–1681. [PubMed] [Google Scholar]
- Godal T., Myklestad B., Samuel D. R., Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971 Dec;9(6):821–831. [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M., Whittle H. C., Molyneux D. H. Immunosuppression in Gambian trypanosomiasis. Trans R Soc Trop Med Hyg. 1973;67(6):846–850. doi: 10.1016/0035-9203(73)90013-8. [DOI] [PubMed] [Google Scholar]
- Haba S., Hamaoka T., Takatsu K., Kitagawa M. Selective suppression of T-cell activity in tumor-bearing mice and its improvement by lentinan, a potent anti-tumor polysaccharide. Int J Cancer. 1976 Jul 15;18(1):93–104. doi: 10.1002/ijc.2910180113. [DOI] [PubMed] [Google Scholar]
- Heilman D. H., McFarland W. Inhibition of tuberculin-induced mitogenesis in cultures of lymphocytes from tuberculous donors. Int Arch Allergy Appl Immunol. 1966;30(1):58–66. doi: 10.1159/000229793. [DOI] [PubMed] [Google Scholar]
- Hsu C. K., Hsu S. H., Whitney R. A., Jr, Hansen C. T. Immunopathology of schistosomiasis in athymic mice. Nature. 1976 Jul 29;262(5567):397–399. doi: 10.1038/262397a0. [DOI] [PubMed] [Google Scholar]
- Inman J. K., Dintzis H. M. The derivatization of cross-linked polyacrylamide beads. Controlled introduction of functional groups for the preparation of special-purpose, biochemical adsorbents. Biochemistry. 1969 Oct;8(10):4074–4082. doi: 10.1021/bi00838a026. [DOI] [PubMed] [Google Scholar]
- James S. L., Colley D. G. Eosinophils and immune mechanisms: production of the lymphokine eosinophil stimulation promoter (ESP) in vitro by isolated intact granulomas. J Reticuloendothel Soc. 1975 Nov;18(5):283–293. [PubMed] [Google Scholar]
- Kasdon E. J., Schlossman S. F. An experimental model of pulmonary arterial granulomatous inflammation. Am J Pathol. 1973 Jun;71(3):365–372. [PMC free article] [PubMed] [Google Scholar]
- Kellermeyer R. W., Warren K. S. The role of chemical mediators in the inflammatory response induced by foreign bodies: comparison with the schistosome egg granuloma. J Exp Med. 1970 Jan 1;131(1):21–39. doi: 10.1084/jem.131.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITT M. Studies in experimental eosinophilia. V. Eosinophils in lynph nodes of guinea pigs following primary antigenic stimulation. Am J Pathol. 1963 May;42:529–549. [PMC free article] [PubMed] [Google Scholar]
- Levene G. M., Turk J. L., Wright D. J., Grimble A. G. Reduced lymphocyte transformation due to a plasma factor in patients with active syphilis. Lancet. 1969 Aug 2;2(7614):246–247. doi: 10.1016/s0140-6736(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Strickland G. T., Warren K. S. Toxoplasmosis and the host-parasite relationship in murine schistosomiasis mansoni. J Infect Dis. 1977 Mar;135(3):408–413. doi: 10.1093/infdis/135.3.408. [DOI] [PubMed] [Google Scholar]
- Ottesen E. A., Hiatt R. A., Cheever A. W., Sotomayor Z. R., Neva F. A. The acquisition and loss of antigen-specific cellular immune responsiveness in acute and chronic schistosomiasis in man. Clin Exp Immunol. 1978 Jul;33(1):37–47. [PMC free article] [PubMed] [Google Scholar]
- Ottesen E. A., Weller P. F., Heck L. Specific cellular immune unresponsiveness in human filariasis. Immunology. 1977 Sep;33(3):413–421. [PMC free article] [PubMed] [Google Scholar]
- Pelley R. P., Ruffier J. J., Warren K. S. Suppressive effect of a chronic helminth infection, schistosomiasis mansoni, on the in vitro responses of spleen and lymph node cells to the T cell mitogens phytohemagglutinin and concanavalin A. Infect Immun. 1976 Apr;13(4):1176–1183. doi: 10.1128/iai.13.4.1176-1183.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. M., DiConza J. J., Gold J. A., Reid W. A. Schistosomiasis in the congenitally athymic (nude) mouse. I. Thymic dependency of eosinophilia, granuloma formation, and host morbidity. J Immunol. 1977 Feb;118(2):594–599. [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Slauson D. O., Dahlstrom M. A. The pulmonary inflammatory response. Cellular events in experimental pulmonary arterial hypersensitivity disease. Am J Pathol. 1975 Apr;79(1):119–130. [PMC free article] [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965 Nov;55(4):695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Parker D. Granuloma formation in normal guinea pigs injected intradermally with aluminum and zirconium compounds. J Invest Dermatol. 1977 Jun;68(6):336–340. doi: 10.1111/1523-1747.ep12496358. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Benacerraf B. Immunologic events in experimental hypersensitivity granulomas. Am J Pathol. 1973 Jun;71(3):349–364. [PMC free article] [PubMed] [Google Scholar]
- Von Lichtenberg F., Erickson D. G., Sadun E. H. Comparative histopathology of schistosome granulomas in the hamster. Am J Pathol. 1973 Aug;72(2):149–178. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S., Boros D. L., Hang L. M., Mahmoud A. A. The Schistosoma japonicum egg granuloma. Am J Pathol. 1975 Aug;80(2):279–294. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O., Cowan R. B. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967 Nov;51(5):735–756. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O. Granuloma formation around Schistosoma mansoni, S. HAEMATOBIUM, AND S. japonicum eggs. Size and rate of development, cellular composition, cross-sensitivity, and rate of egg destruction. Am J Trop Med Hyg. 1970 Mar;19(2):292–304. doi: 10.4269/ajtmh.1970.19.292. [DOI] [PubMed] [Google Scholar]
- Warren K. S., Grove D. I., Pelley R. P. The Schistosoma japonicum egg granuloma. II. Cellular composition, granuloma size, and immunologic concomitants. Am J Trop Med Hyg. 1978 Mar;27(2 Pt 1):271–275. doi: 10.4269/ajtmh.1978.27.271. [DOI] [PubMed] [Google Scholar]
- Zeitz S. J., Ostrow J. H., Van Arsdel P. P., Jr Humoral and cellular immunity in the anergic tuberculosis patient. A prospective study. J Allergy Clin Immunol. 1974 Jan;53(1):20–26. doi: 10.1016/0091-6749(74)90095-5. [DOI] [PubMed] [Google Scholar]
- von LICHTENBERG Host response to eggs of S. mansoni. I. Granuloma formation in the unsensitized laboratory mouse. Am J Pathol. 1962 Dec;41:711–731. [PMC free article] [PubMed] [Google Scholar]
- von Lichtenberg F. Experimental approaches to human schistosomiasis. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):79–87. doi: 10.4269/ajtmh.1977.26.79. [DOI] [PubMed] [Google Scholar]