Abstract
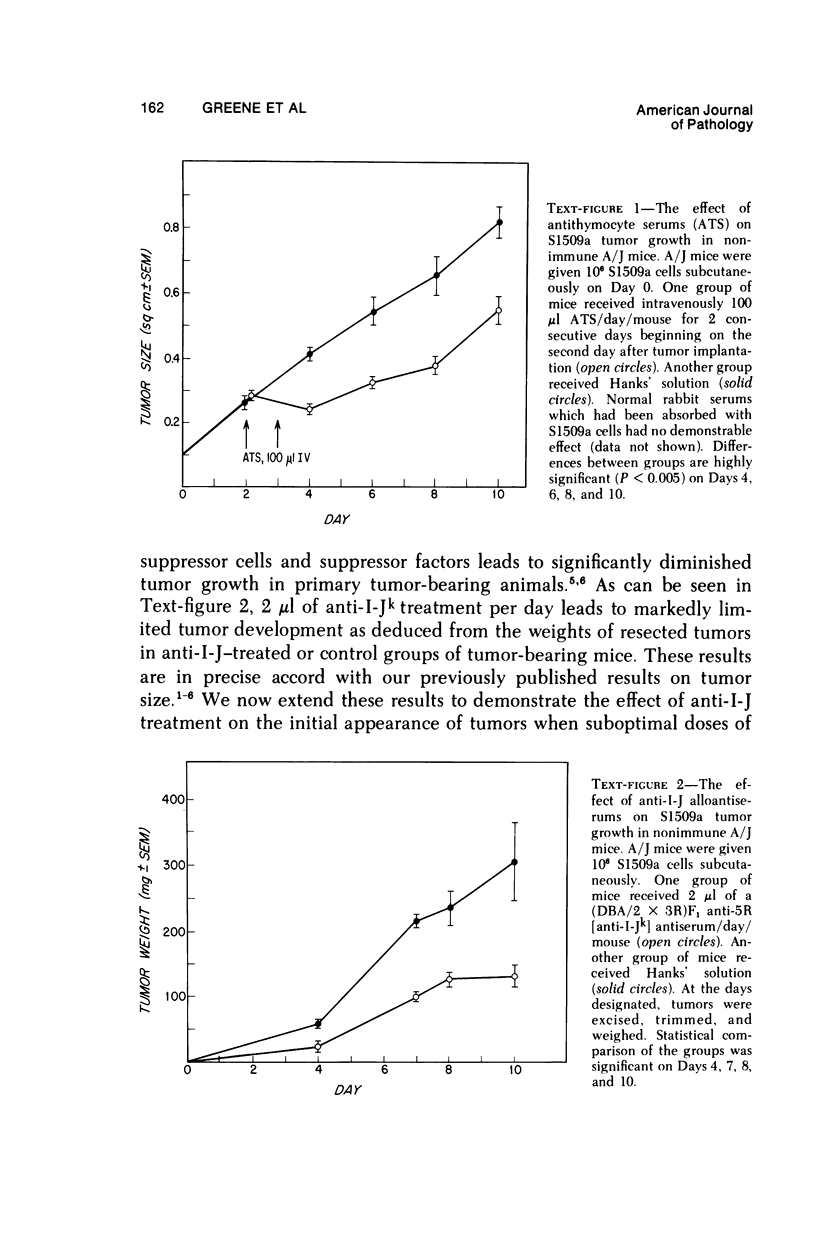
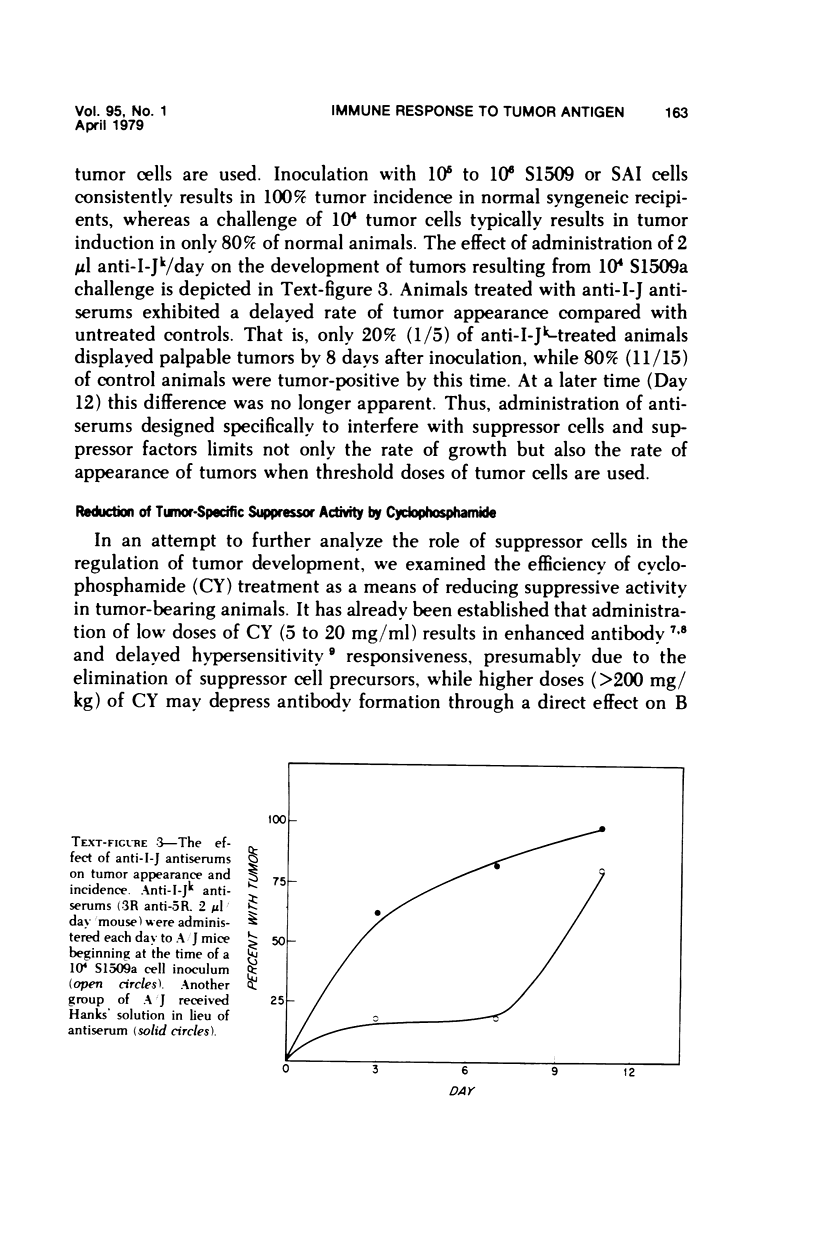
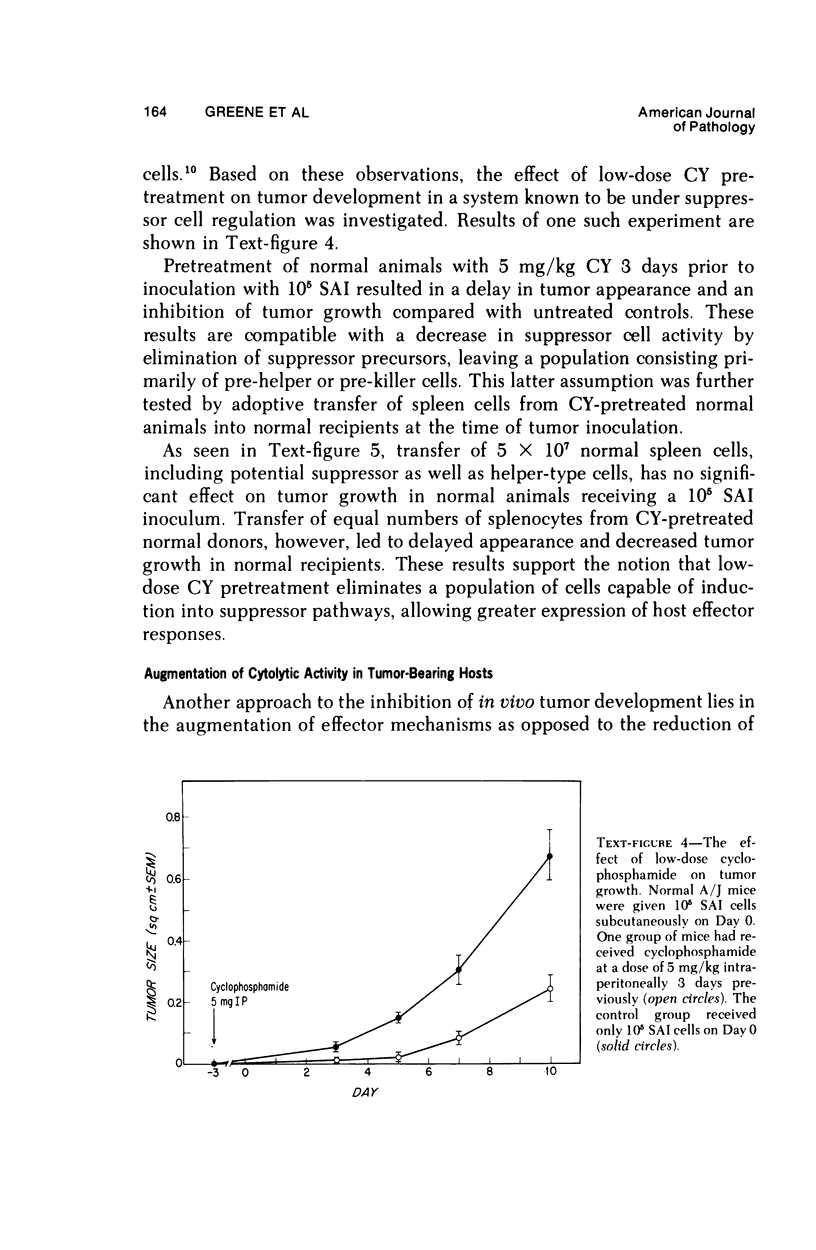
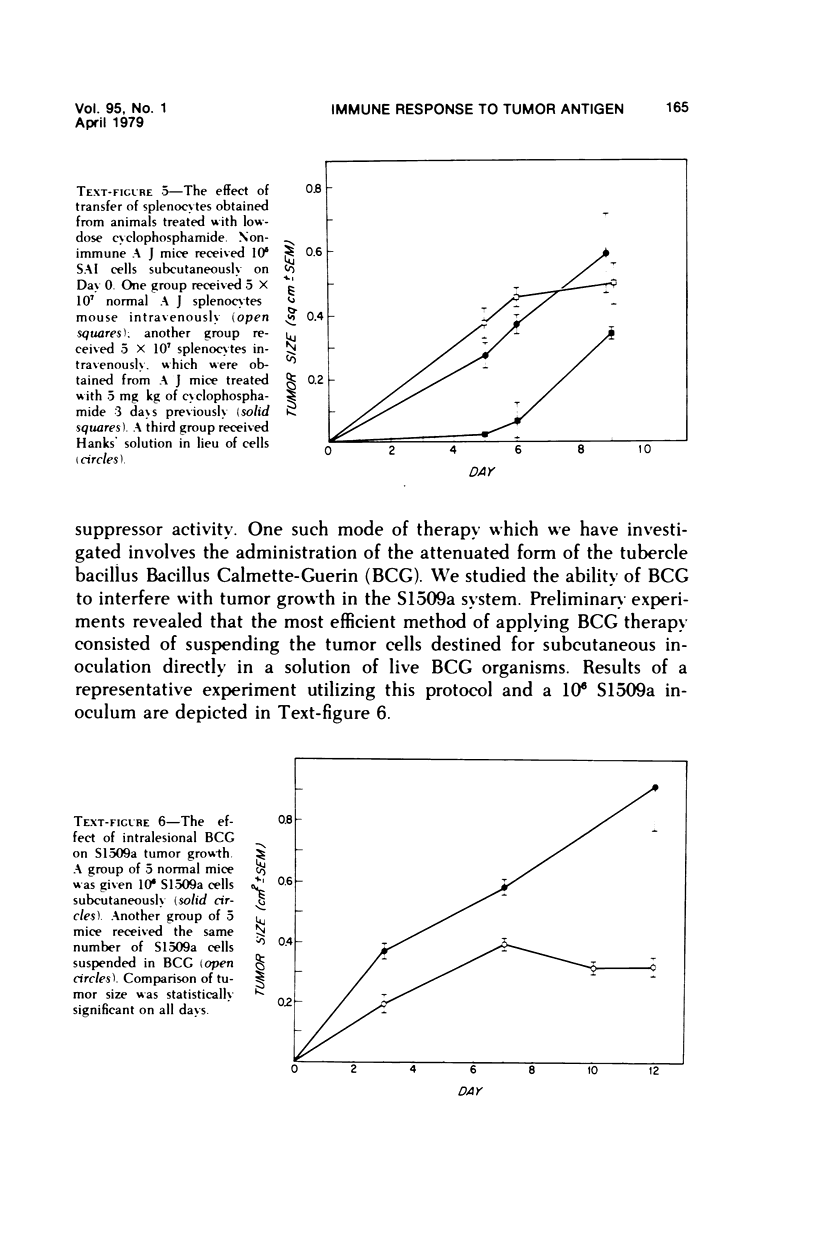
Reduction of syngeneic tumor growth in primary tumor-bearing murine hosts has been accomplished using a variety of treatments designed to decrease endogenous suppressor cell activity or augment host effector responses. Selective interference with suppressor cell function can be achieved by in vivo administration of anti-thymocyte serums at critical times during the early stages of tumor development or by continuous treatment with antiserums directed to interact with I-J determinants on suppressor cells or suppressor factors. This later mode of therapy also results in a delay in tumor appearance when suboptimal doses of tumor are given. Preferential diminution of suppressor cell precursor activity has also been observed by pretreatment of tumor recipients with low doses of cyclophosphamide. Normal animals so treated are capable of adoptively transferring primarily helper-type activity to tumor-bearing recipients. Decreased tumor growth and prolonged host survival have also been achieved using BCG as a means of augmenting host effector potential. Thus, it is possible to inhibit tumor development in a murine model by modes of immunotherapy which may be relevant to the early treatment of certain human neoplasms.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenase P. W., Hayden B. J., Gershon R. K. Augmentation of delayed-type hypersensitivity by doses of cyclophosphamide which do not affect antibody responses. J Exp Med. 1975 Mar 1;141(3):697–702. doi: 10.1084/jem.141.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos H., Galanaud P., Devinsky O., Maillot M. C., Dormont J. Enhancing effect of low dose cyclophosphamide treatment on the in vitro antibody response. Eur J Immunol. 1977 Oct;7(10):679–684. doi: 10.1002/eji.1830071005. [DOI] [PubMed] [Google Scholar]
- Eardley D. D., Hugenberger J., McVay-Boudreau L., Shen F. W., Gershon R. K., Cantor H. Immunoregulatory circuits among T-cell sets. I. T-helper cells induce other T-cell sets to exert feedback inhibition. J Exp Med. 1978 Apr 1;147(4):1106–1115. doi: 10.1084/jem.147.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Greene M. I., Sehon A. H. Regualtion of the immune response to tumor antigens. I. Immunosuppressor cells in tumor-bearing hosts. J Immunol. 1976 Mar;116(3):791–799. [PubMed] [Google Scholar]
- Fujimoto S., Greene M. I., Sehon A. H. Regulation of the immune response to tumor antigens. II. The nature of immunosuppressor cells in tumor-bearing hosts. J Immunol. 1976 Mar;116(3):800–806. [PubMed] [Google Scholar]
- Germain R. N., Williams R. M., Benacerraf B. Specific and nonspecific antitumor immunity. II. Macrophage-mediated nonspecific effector activity induced by BCG and similar agents. J Natl Cancer Inst. 1975 Mar;54(3):709–720. [PubMed] [Google Scholar]
- Greene M. I., Dorf M. E., Pierres M., Benacerraf B. Reduction of syngeneic tumor growth by an anti-I-J-alloantiserum. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5118–5121. doi: 10.1073/pnas.74.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M. I., Fujimoto S., Sehon A. H. Regulation of the immune response to tumor antigens. III. Characterization of thymic suppressor factor(s) produced by tumor-bearing hosts. J Immunol. 1977 Aug;119(2):757–764. [PubMed] [Google Scholar]
- Haagensen D. E., Jr, Roloson G., Collins J. J., Wells S. A., Jr, Bolognesi D. P., Hansen H. J. Immunologic control of the ascites of murine adenocarcinoma 755. I. Protection with syngeneic immune serum or lymphoid cells. J Natl Cancer Inst. 1978 Jan;60(1):131–139. doi: 10.1093/jnci/60.1.131. [DOI] [PubMed] [Google Scholar]
- Hellström I., Hellström K. E. Cyclophosphamide delays 3-methylcholanthrene sarcoma induction in mice. Nature. 1978 Sep 14;275(5676):129–130. doi: 10.1038/275129a0. [DOI] [PubMed] [Google Scholar]
- Perry L. L., Benacerraf B., McCluskey R. T., Greene M. I. Enhanced syngeneic tumor destruction by in vivo inhibition of suppressor T cells using anti-I-J alloantiserum. Am J Pathol. 1978 Aug;92(2):491–506. [PMC free article] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., David C. S. Properties of the antigen-specific suppressive T-cell factor in the regulation of antibody response of the mouse. IV. Special subregion assignment of the gene(s) that codes for the suppressive T-cell factor in the H-2 histocompatibility complex. J Exp Med. 1976 Sep 1;144(3):713–725. doi: 10.1084/jem.144.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Parker D., Poulter L. W. Functional aspects of the selective depletion of lymphoid tissue by cyclophosphamide. Immunology. 1972 Oct;23(4):493–501. [PMC free article] [PubMed] [Google Scholar]
- Waltenbaugh C., Thèze J., Kapp J. A., Benacerraf B. Immunosuppressive factor(s) specific for L-glutamic acid50-L-tyrosine50 (GT). III. Generation of suppressor T cells by a suppressive extract derived from GT-primed lymphoid cells. J Exp Med. 1977 Oct 1;146(4):970–985. doi: 10.1084/jem.146.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]


