Abstract
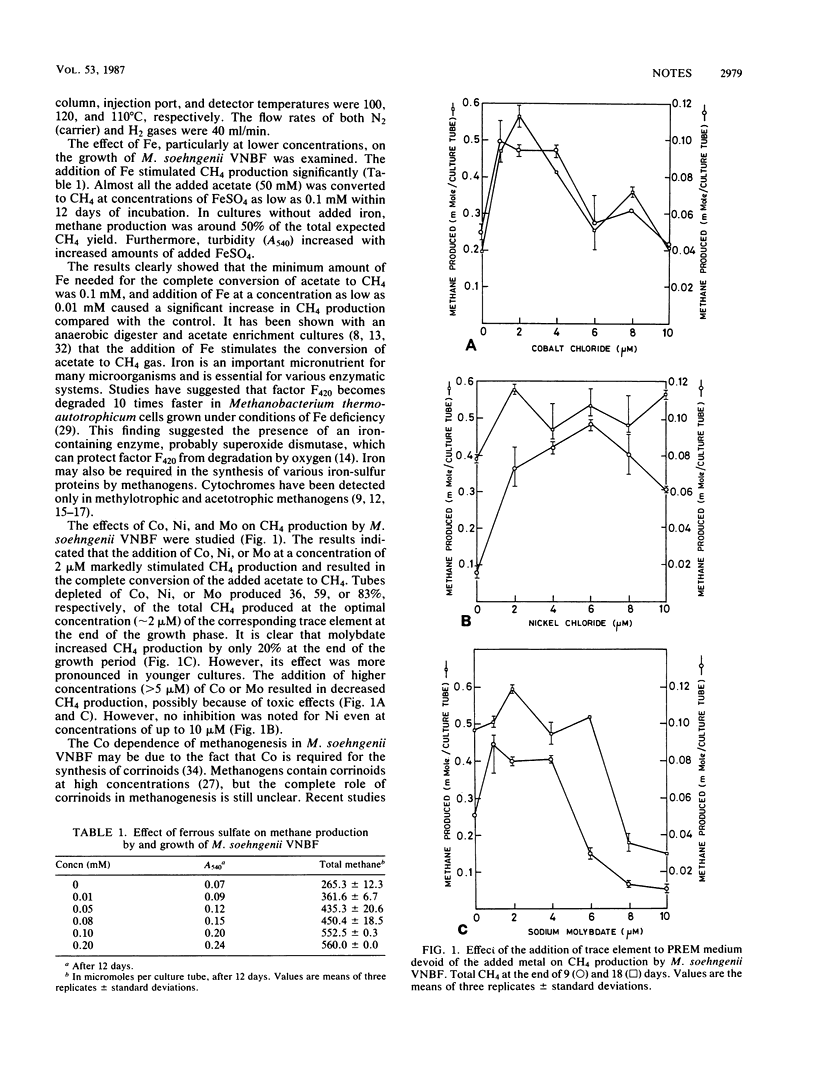
Methane production by Methanothrix soehngenii VNBF grown on acetate (50 mM) as the sole carbon and energy source was influenced by the addition of Fe, trace elements, and pesticides. The addition of Fe and trace elements significantly enhanced the rate of CH4 production. The addition of pesticides in the early growth phase caused complete inhibition. However, less inhibition was noted when pesticides were added during early exponential growth phase. Addition to culture tubes of Co, Ni, or Mo at 2 μM produced 64, 41, or 17%, respectively, more CH4 than that produced in tubes lacking the corresponding trace element. A concentration of more than 5 μM of these trace elements in the medium resulted in decreased CH4 production, presumably because of toxic effects.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Diekert G., Klee B., Thauer R. K. Nickel, a component of factor F430 from Methanobacterium thermoautotrophicum. Arch Microbiol. 1980 Jan;124(1):103–106. doi: 10.1007/BF00407036. [DOI] [PubMed] [Google Scholar]
- Ellefson W. L., Whitman W. B. The role of nickel in methanogenic bacteria. Basic Life Sci. 1982;19:403–414. doi: 10.1007/978-1-4684-4142-0_30. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. ATP activation and properties of the methyl coenzyme M reductase system in Methanobacterium thermoautotrophicum. J Bacteriol. 1978 Sep;135(3):851–857. doi: 10.1128/jb.135.3.851-857.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban D. J., van den Berg L. Effect of iron on conversion of acetic acid to methane during methanogenic fermentations. J Appl Bacteriol. 1979 Aug;47(1):153–159. doi: 10.1111/j.1365-2672.1979.tb01179.x. [DOI] [PubMed] [Google Scholar]
- Jarrell K. F., Colvin J. R., Sprott G. D. Spontaneous protoplast formation in Methanobacterium bryantii. J Bacteriol. 1982 Jan;149(1):346–353. doi: 10.1128/jb.149.1.346-353.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. F., Sprott G. D. Nickel transport in Methanobacterium bryantii. J Bacteriol. 1982 Sep;151(3):1195–1203. doi: 10.1128/jb.151.3.1195-1203.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T. W., Lancaster J. R., Jr, Fridovich I. Isolation and characterization of the iron-containing superoxide dismutase of Methanobacterium bryantii. Arch Biochem Biophys. 1981 Aug;210(1):140–148. doi: 10.1016/0003-9861(81)90174-0. [DOI] [PubMed] [Google Scholar]
- Krzycki J. A., Zeikus J. G. Characterization and purification of carbon monoxide dehydrogenase from Methanosarcina barkeri. J Bacteriol. 1984 Apr;158(1):231–237. doi: 10.1128/jb.158.1.231-237.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn W., Fiebig K., Walther R., Gottschalk G. Presence of a cytochrome b559 in Methanosarcina barkeri. FEBS Lett. 1979 Sep 15;105(2):271–274. doi: 10.1016/0014-5793(79)80627-4. [DOI] [PubMed] [Google Scholar]
- Lal R. Accumulation, metabolism, and effects of organophosphorus insecticides on microorganisms. Adv Appl Microbiol. 1982;28:149–200. doi: 10.1016/s0065-2164(08)70235-1. [DOI] [PubMed] [Google Scholar]
- Lal R., Saxena D. M. Accumulation, metabolism, and effects of organochlorine insecticides on microorganisms. Microbiol Rev. 1982 Mar;46(1):95–127. doi: 10.1128/mr.46.1.95-127.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah R. A., Smith M. R., Baresi L. Studies on an acetate-fermenting strain of Methanosarcina. Appl Environ Microbiol. 1978 Jun;35(6):1174–1184. doi: 10.1128/aem.35.6.1174-1184.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Wolfe R. S. Inhibition of methanogenesis by DDT. Nature. 1971 Dec 31;234(5331):551–552. doi: 10.1038/234551a0. [DOI] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Changes in proportions of acetate and carbon dioxide used as methane precursors during the anaerobic digestion of bovine waste. Appl Environ Microbiol. 1978 Apr;35(4):648–654. doi: 10.1128/aem.35.4.648-654.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray W. D., van den Berg L. Effects of nickel, cobalt, and molybdenum on performance of methanogenic fixed-film reactors. Appl Environ Microbiol. 1981 Sep;42(3):502–505. doi: 10.1128/aem.42.3.502-505.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel G. B., Khan A. W., Roth L. A. Optimum levels of sulphate and iron for the cultivation of pure cultures of methanogens in synthetic media. J Appl Bacteriol. 1978 Dec;45(3):347–356. doi: 10.1111/j.1365-2672.1978.tb04235.x. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Moura I., Moura J. J., Santos M. H., Xavier A. V., LeGall J., Scandellari M. Role of vitamin B12 in methyl transfer for methane biosynthesis by Methanosarcina barkeri. Science. 1982 Apr 16;216(4543):303–305. doi: 10.1126/science.7063887. [DOI] [PubMed] [Google Scholar]
- van den Berg L., Patel G. B., Clark D. S., Lentz C. P. Factors affecting rate of methane formation from acetic acid by enriched methanogenic cultures. Can J Microbiol. 1976 Sep;22(9):1312–1319. doi: 10.1139/m76-194. [DOI] [PubMed] [Google Scholar]


