Abstract
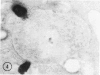
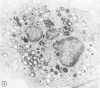
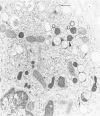
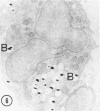
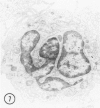
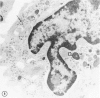
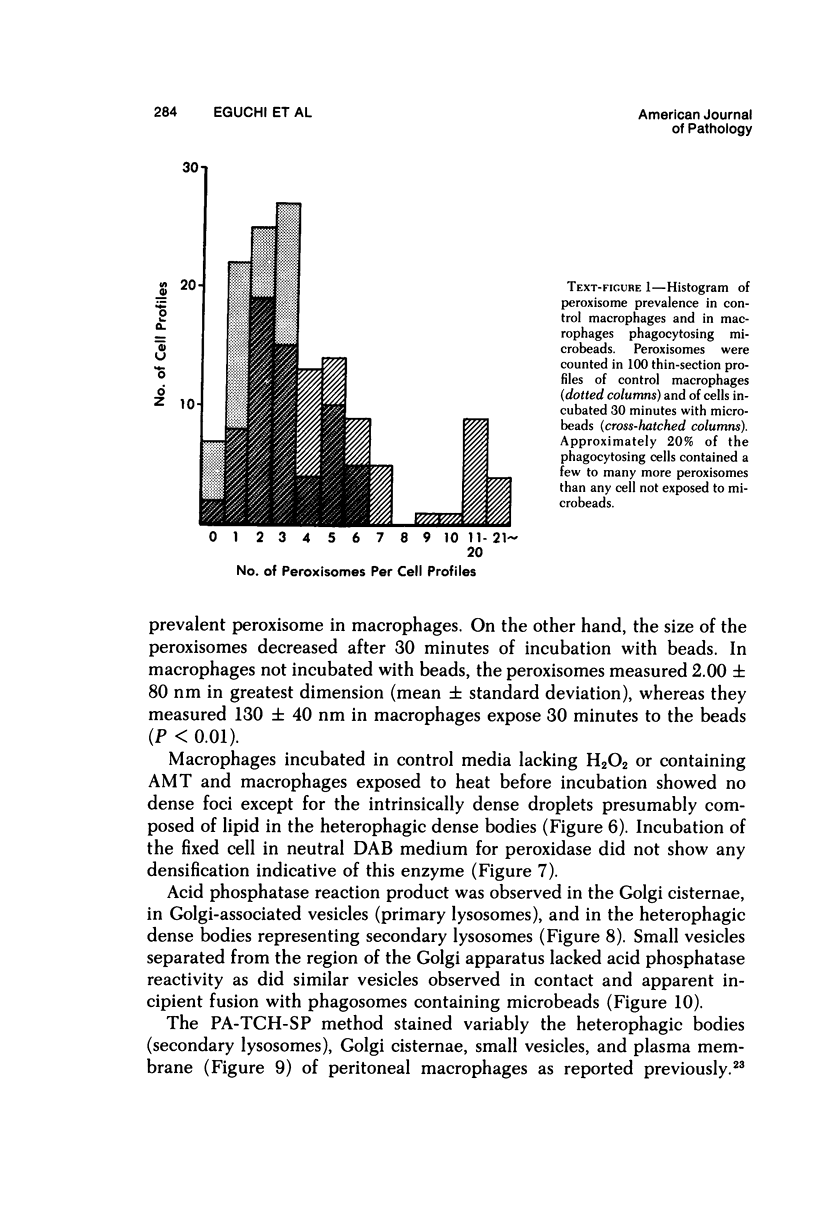
The peroxisomes of resident macrophages in the rat peritoneal cavity were examined during the phagocytosis of latex microbeads, employing the akaline diaminobenzidine (DAB) technique. Peroxisomes generally were located in close proximity to phagosomes and were often observed in a process of apparent fusion with phagosomes. Cytochemical evidence was also obtained for discharge of catalase from peroxisomes to phagosomes. The profiles indicating fusion were observed after 10 minutes of incubation with microbeads. The number of peroxisomes was increased in macrophage profiles examined 30 minutes after exposure to microbeads. Acid phosphatase was localized in small vesicles that were distinct from peroxisomes, and peroxidase was not demonstrable in peroxisomes. A method for ultrastructural localization of periodate reactive complex carbohydrate demonstrated glycoproteins in numerous small vesicles or granules, some of which possibly represented peroxisomers. The possible function of peroxisomes during phagocytosis in rat peritoneal macrophages is considered.
Full text
PDF






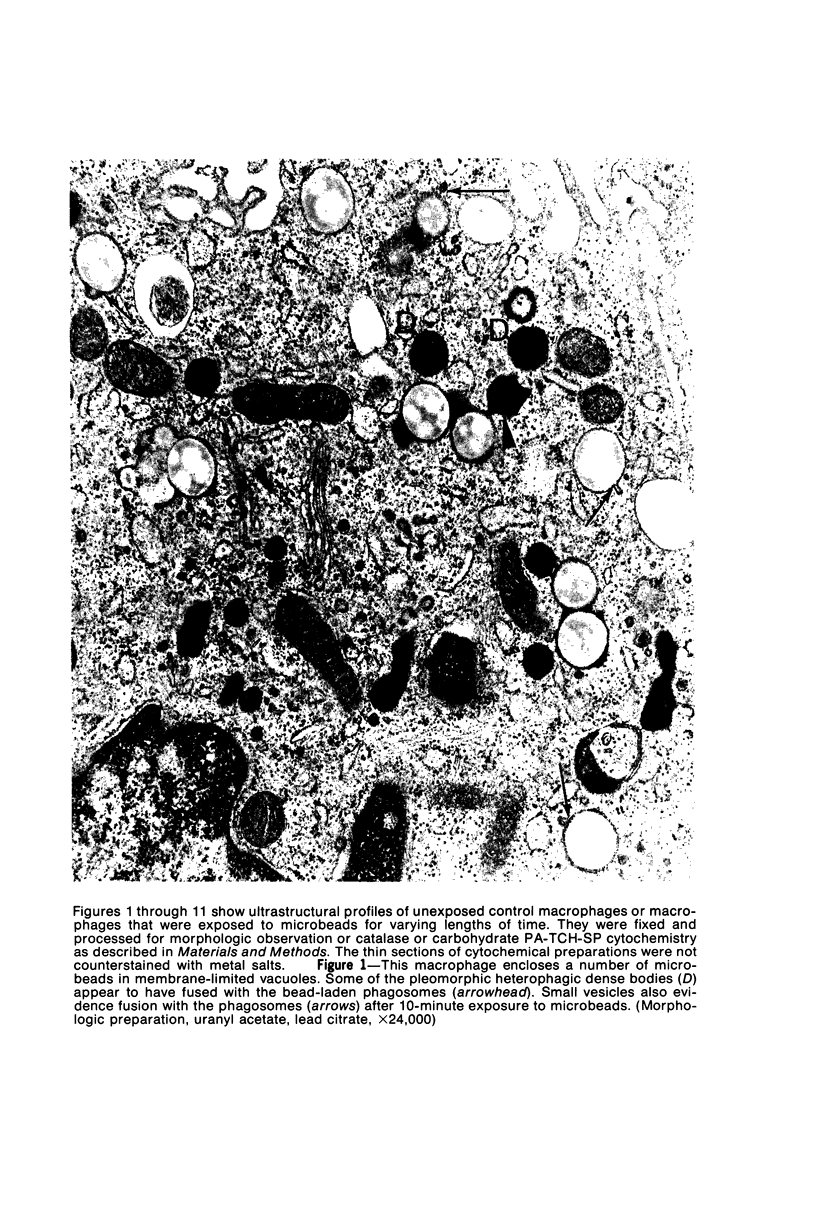
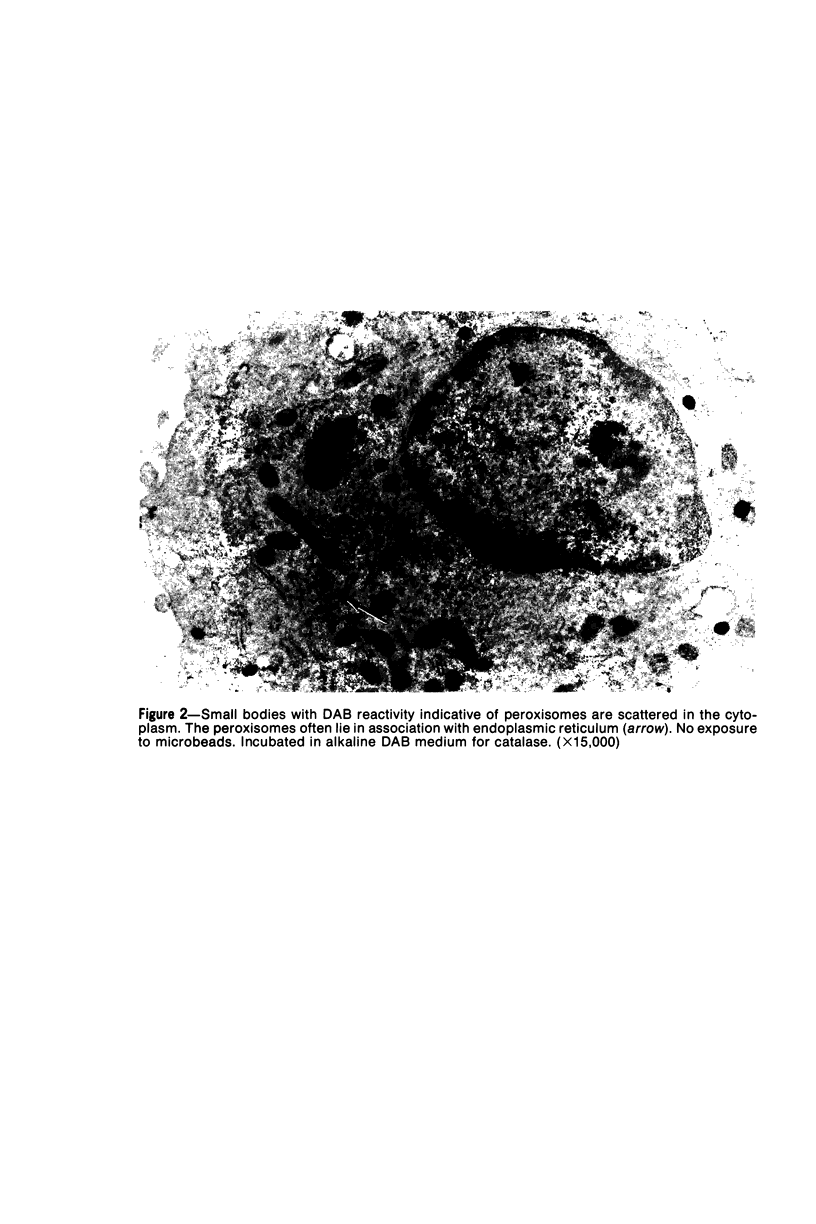
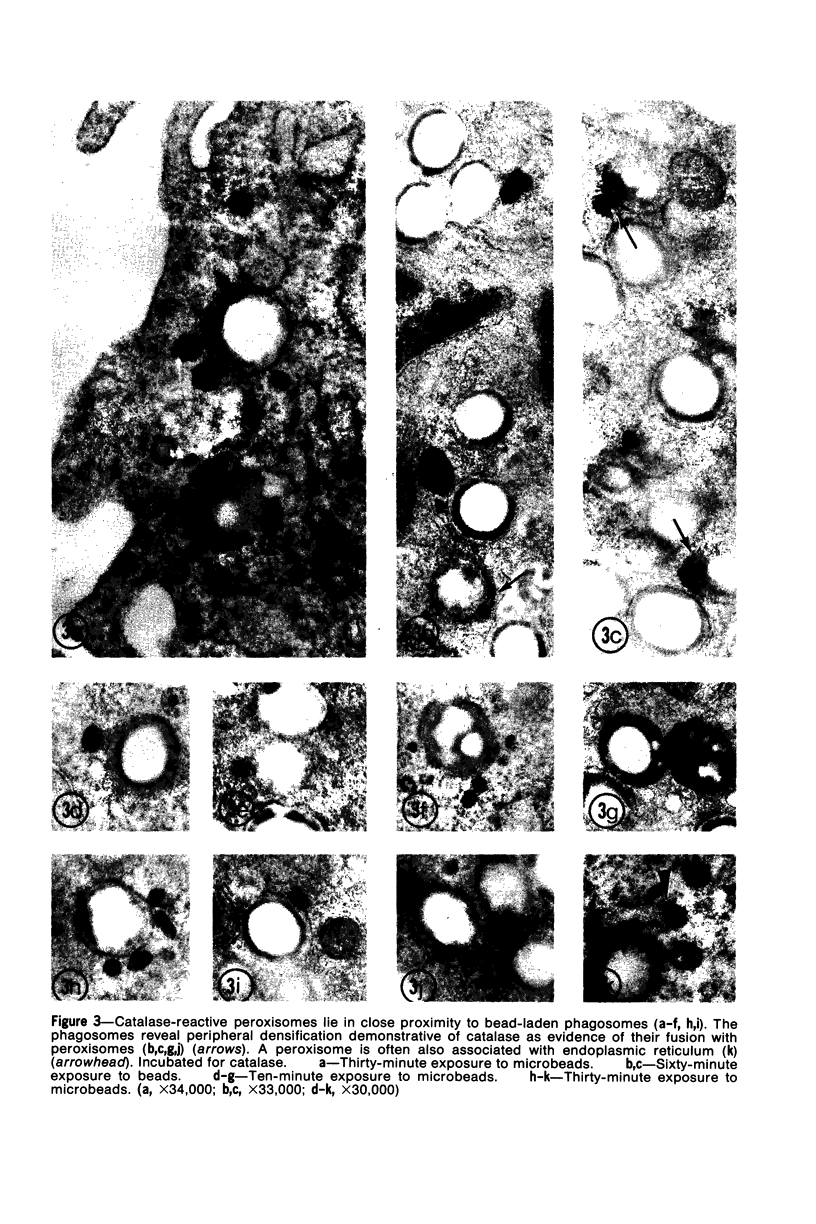
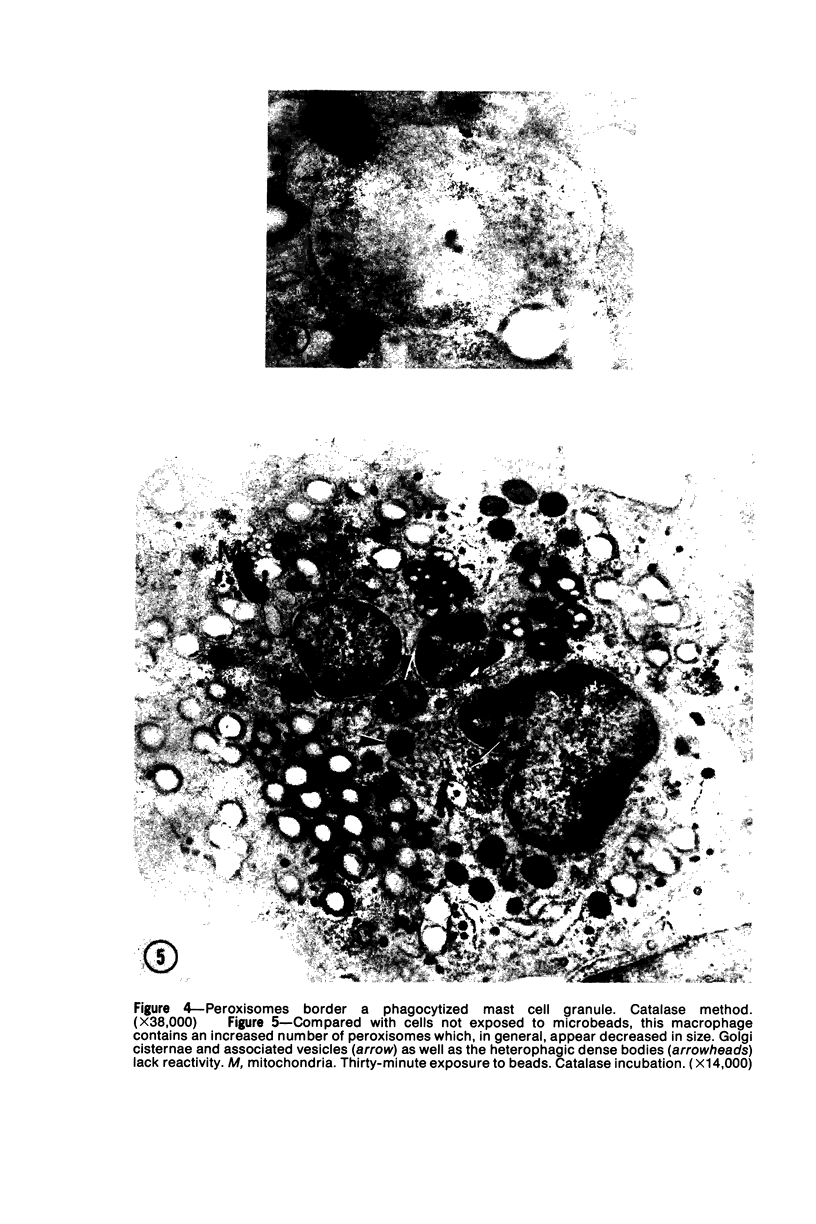
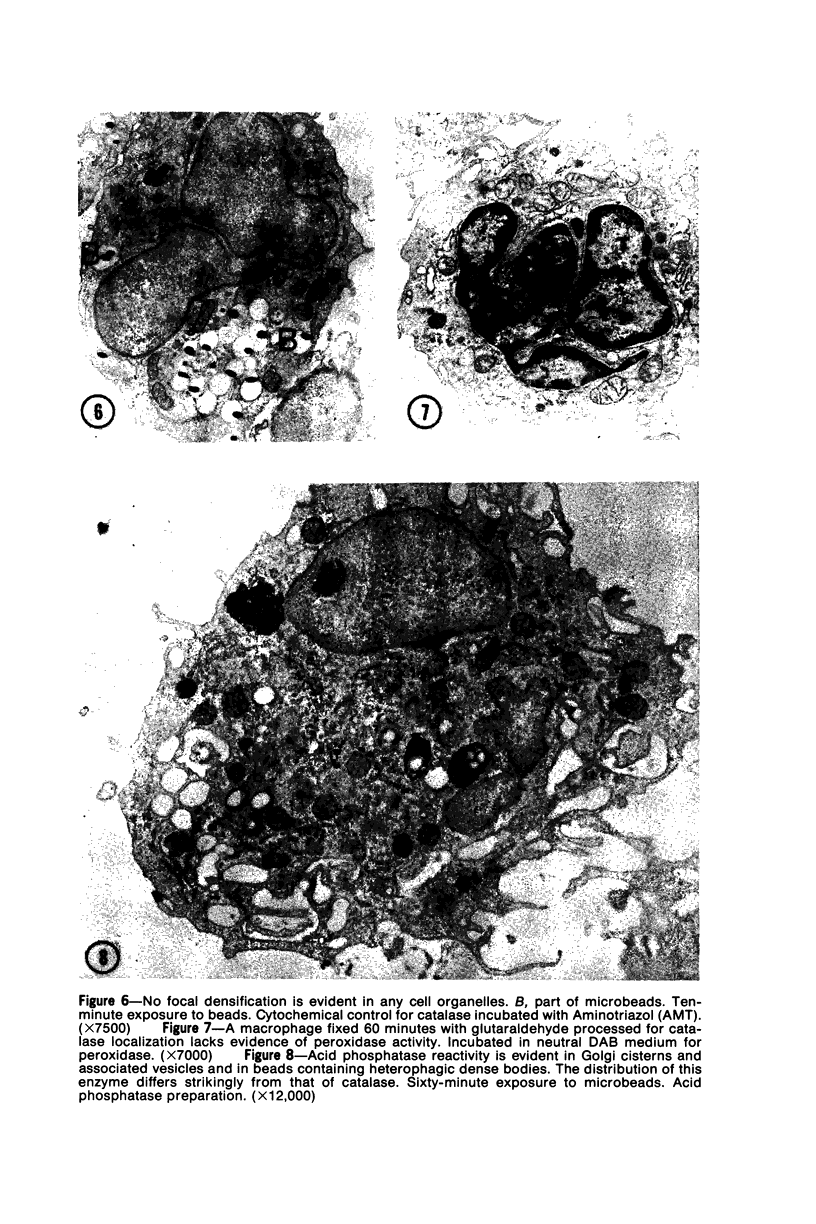
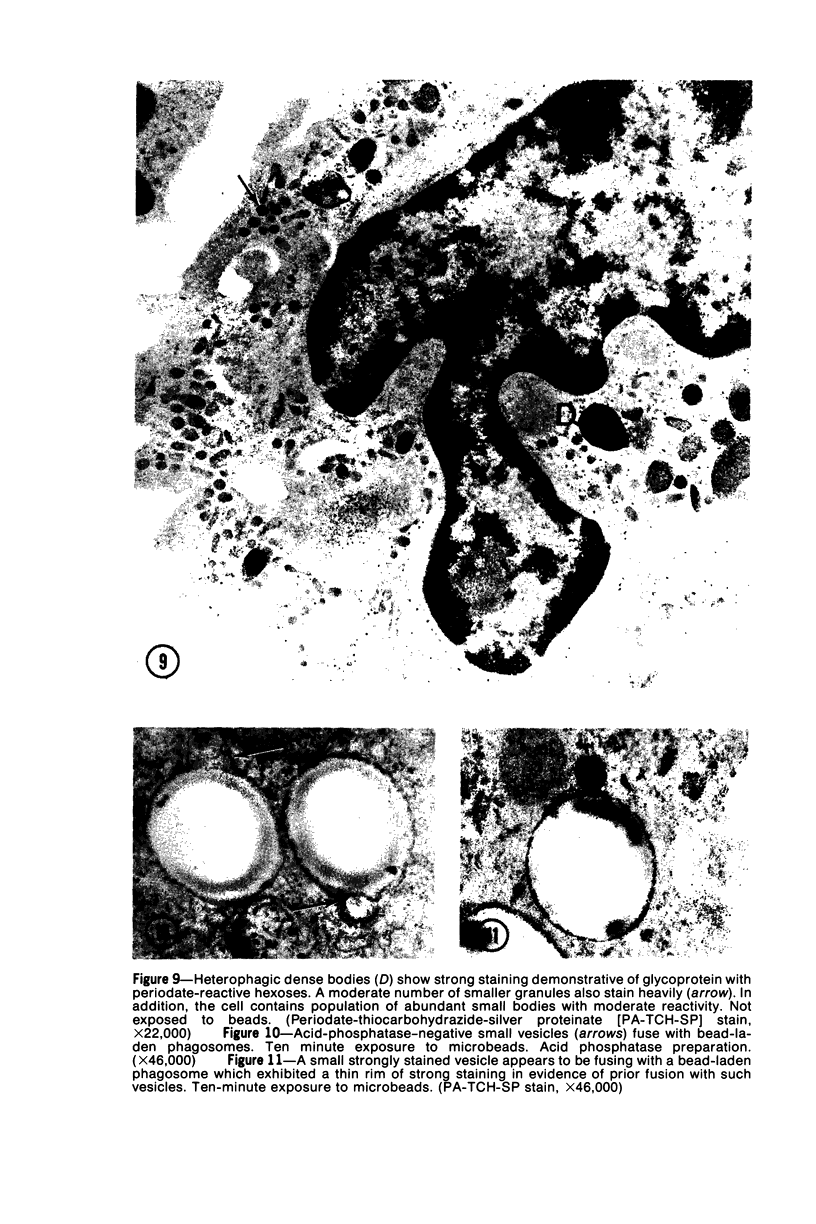
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodel P. T., Nichols B. A., Bainton D. F. Appearance of peroxidase reactivity within the rough endoplasmic reticulum of blood monocytes after surface adherence. J Exp Med. 1977 Feb 1;145(2):264–274. doi: 10.1084/jem.145.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton-Gorius J., Guichard J. Fine structural and cytochemical identification of microperoxisomes in developing human erythrocytic cells. Am J Pathol. 1975 Jun;79(3):523–536. [PMC free article] [PubMed] [Google Scholar]
- Cline M. J., Sumner M. A. Bone marrow macrophage precursors. I. Some functional characteristics of the early cells of the mouse macrophage series. Blood. 1972 Jul;40(1):62–69. [PubMed] [Google Scholar]
- Daems W. T., Poelman R. E., Brederoo P., van Lohuzen E. J. Peroxidatic activity in resident peritoneal macrophages and exudate monocytes of the guinea pig after ingestion of latex particles. J Histochem Cytochem. 1973 Jan;21(1):93–95. doi: 10.1177/21.1.93. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Dounce A. L. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970 Jul;50(3):319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- Fahimi H. D., Gray B. A., Herzog V. K. Cytochemical localization of catalase and peroxidase in sinusoidal cells of rat liver. Lab Invest. 1976 Feb;34(2):192–201. [PubMed] [Google Scholar]
- Feinstein R. N., Savol R., Howard J. B. Conversion of catalatic to peroxidatic activity in livers of normal and acatalasemic mice. Enzymologia. 1971 Dec 31;41(6):345–358. [PubMed] [Google Scholar]
- Gee J. B., Vassallo C. L., Bell P., Kaskin J., Basford R. E., Field J. B. Catalase-dependent peroxidative metabolism in the alveolar macrophage during phagocytosis. J Clin Invest. 1970 Jun;49(6):1280–1287. doi: 10.1172/JCI106340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S., Roheim P. S., Edelstein D., Essner E. Hypolipidemia in a mutant strain of "acatalasemic" mice. Science. 1971 Jul 2;173(3991):65–66. doi: 10.1126/science.173.3991.65. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hruban Z., Vigil E. L., Slesers A., Hopkins E. Microbodies: constituent organelles of animal cells. Lab Invest. 1972 Aug;27(2):184–191. [PubMed] [Google Scholar]
- Jensen M. S., Bainton D. F. Temporal changes in pH within the phagocytic vacuole of the polymorphonuclear neutrophilic leukocyte. J Cell Biol. 1973 Feb;56(2):379–388. doi: 10.1083/jcb.56.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial activity of catalase at acid pH. Proc Soc Exp Biol Med. 1969 Nov;132(2):571–574. doi: 10.3181/00379727-132-34263. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Clem W. H., Luebke R. G. The peroxidase-thiocyanate-hydrogen peroxide antimicrobial system. Biochim Biophys Acta. 1966 Mar 28;117(1):63–72. doi: 10.1016/0304-4165(66)90152-8. [DOI] [PubMed] [Google Scholar]
- Komiyama A., Spicer S. S., Bank H., Farrington J. Induction of autophagic vacuoles in peritoneal cells. J Reticuloendothel Soc. 1975 Mar;17(3):146–161. [PubMed] [Google Scholar]
- Lazarow P. B., de Duve C. The synthesis and turnover of rat liver of rat liver peroxisomes. IV. Biochemical pathway of catalase synthesis. J Cell Biol. 1973 Nov;59(2 Pt 1):491–506. doi: 10.1083/jcb.59.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGroarty E., Tolbert N. E. Enzymes in peroxisomes. J Histochem Cytochem. 1973 Nov;21(11):949–954. doi: 10.1177/21.11.949. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Davis C., Quintana N. Studies on microperoxisomes. II. A cytochemical method for light and electron microscopy. J Histochem Cytochem. 1972 Dec;20(12):1006–1023. doi: 10.1177/20.12.1006. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Davis C., Quintana N. Studies on microperoxisomes. V. Are microperoxisomes ubiquitous in mammalian cells? J Histochem Cytochem. 1973 Aug;21(8):737–755. doi: 10.1177/21.8.737. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M. Microperoxisomes. J Histochem Cytochem. 1973 Nov;21(11):963–966. doi: 10.1177/21.11.963. [DOI] [PubMed] [Google Scholar]
- Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Function of h(2)o(2), myeloperoxidase, and hexose monophosphate shunt enzymes in phagocytizing cells from different species. Infect Immun. 1970 Apr;1(4):338–344. doi: 10.1128/iai.1.4.338-344.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K. Possible properties of microbodies (peroxisomes). Microbody proliferation and hypolipidemic drugs. J Histochem Cytochem. 1973 Nov;21(11):967–971. doi: 10.1177/21.11.967. [DOI] [PubMed] [Google Scholar]
- Robbins D., Fahimi H. D., Cotran R. S. Fine structural cytochemical localization of peroxidase activity in rat peritoneal cells: mononuclear cells, eosinophils and mast cells. J Histochem Cytochem. 1971 Sep;19(9):571–575. doi: 10.1177/19.9.571. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Pollard T. D., Vaughan M. Isolation and properties of phagocytic vesicles. II. Alveolar macrophages. J Clin Invest. 1972 Mar;51(3):604–614. doi: 10.1172/JCI106850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stäubli W., Schweizer W., Suter J., Weibel E. R. The proliferative response of hepatic peroxidomes of neonatal rats to treatment with SU-13 437 (nafenopin). J Cell Biol. 1977 Sep;74(3):665–689. doi: 10.1083/jcb.74.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda D., Reddy J. Microbodies in experimentally altered cells. IX. The fate of microbodies. Am J Pathol. 1972 Jun;67(3):541–554. [PMC free article] [PubMed] [Google Scholar]
- Wisse E. Observations on the fine structure and peroxidase cytochemistry of normal rat liver Kupffer cells. J Ultrastruct Res. 1974 Mar;46(3):393–426. doi: 10.1016/s0022-5320(74)90064-1. [DOI] [PubMed] [Google Scholar]
- van Furth R., Hirsch J. G., Fedorko M. E. Morphology and peroxidase cytochemistry of mouse promonocytes, monocytes, and macrophages. J Exp Med. 1970 Oct 1;132(4):794–812. doi: 10.1084/jem.132.4.794. [DOI] [PMC free article] [PubMed] [Google Scholar]