Abstract
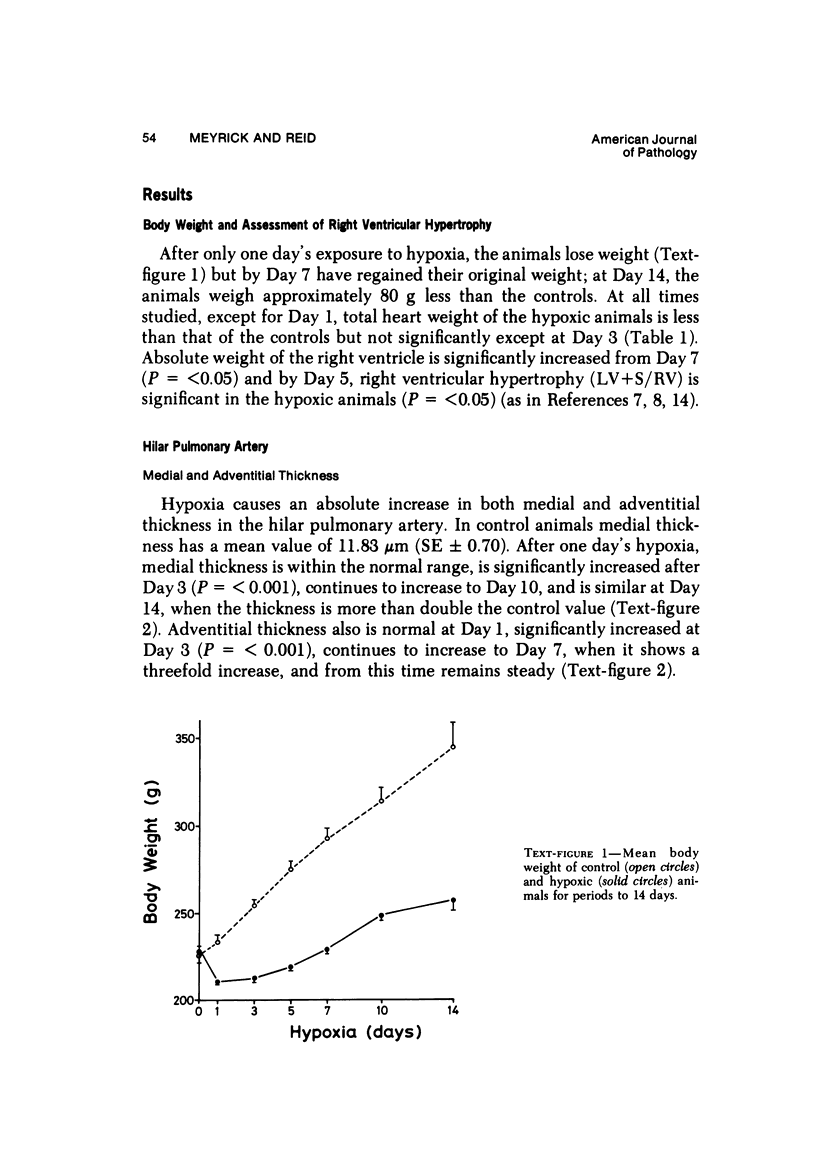
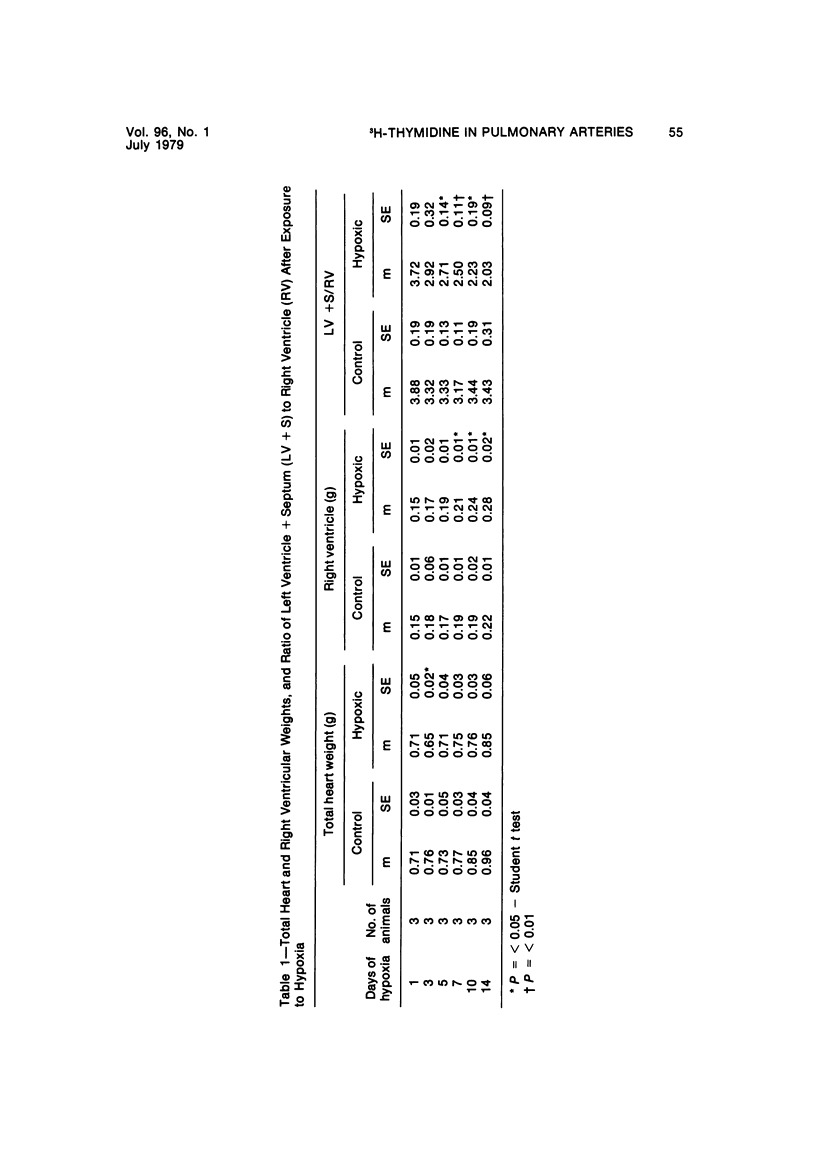
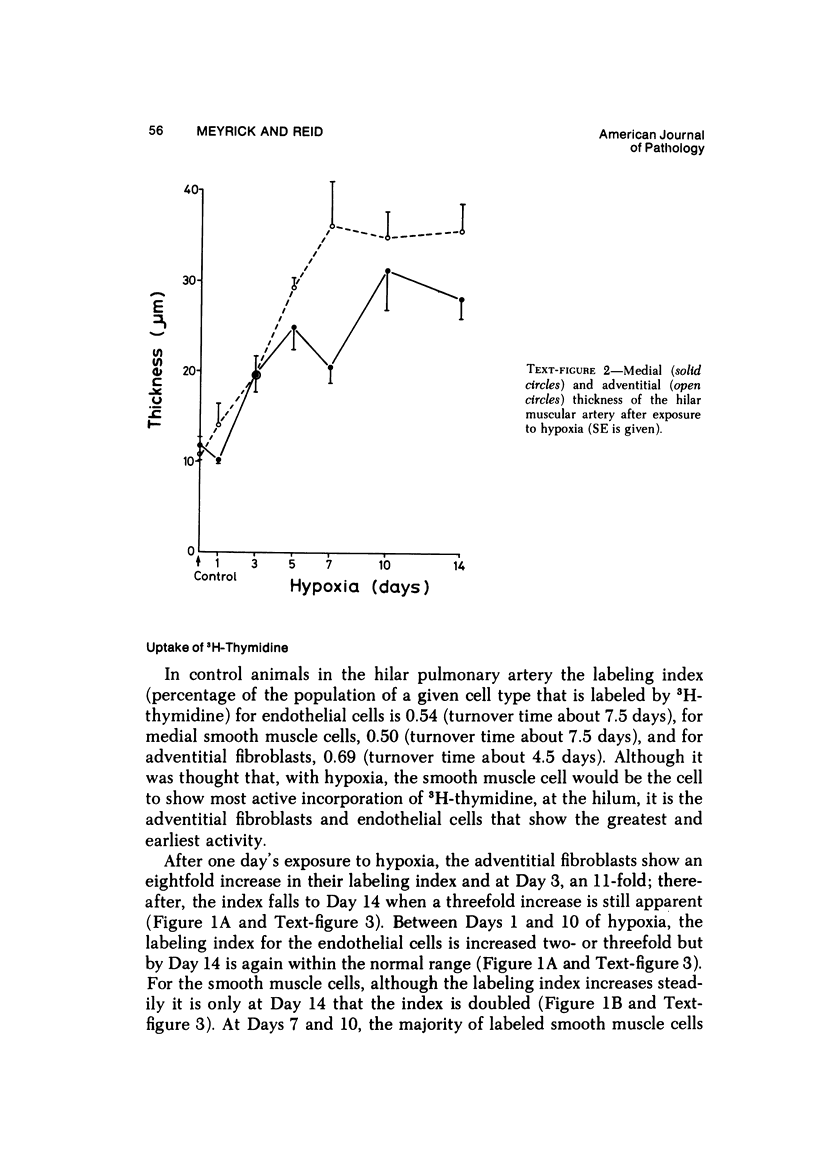
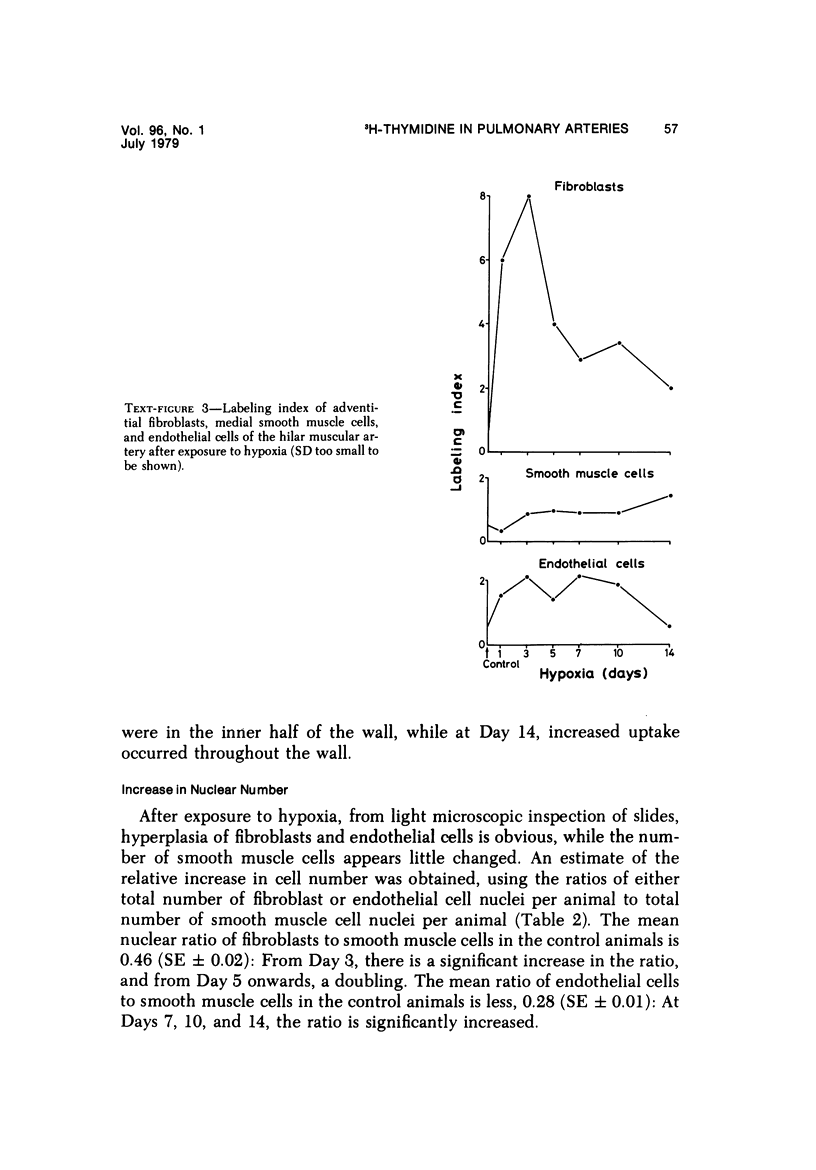
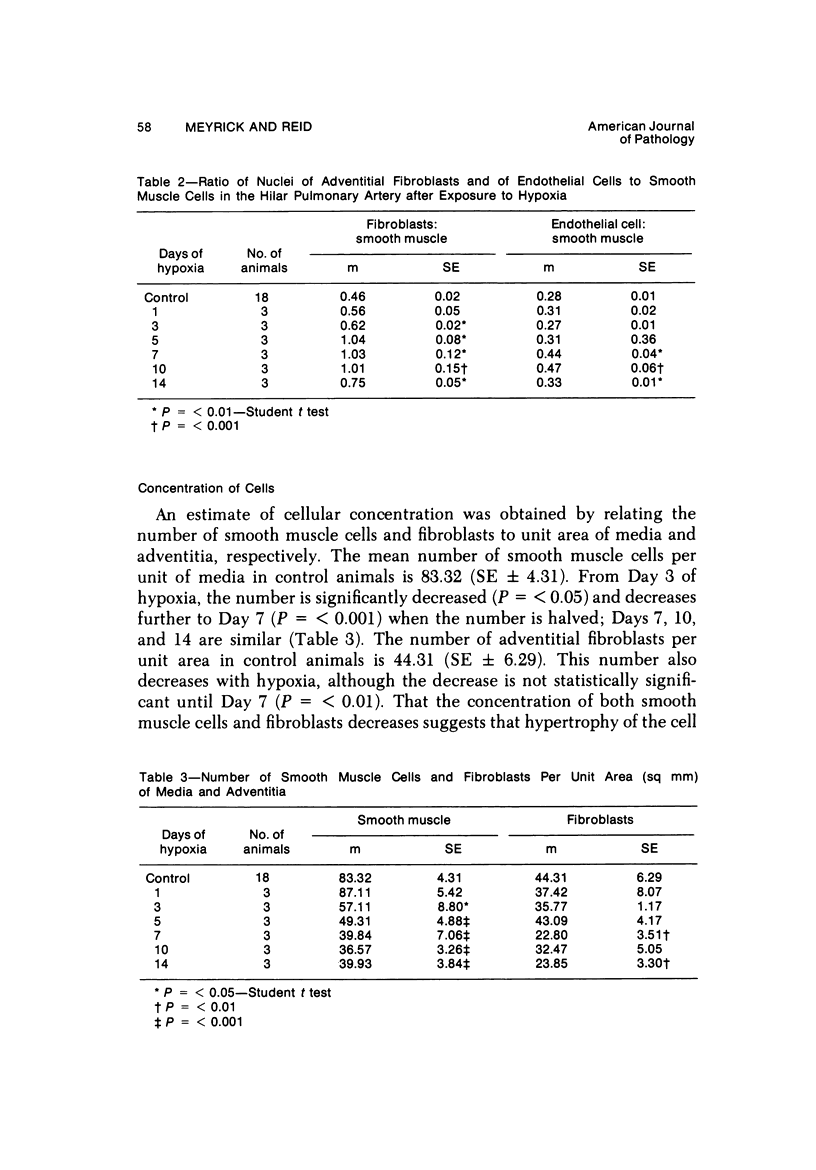
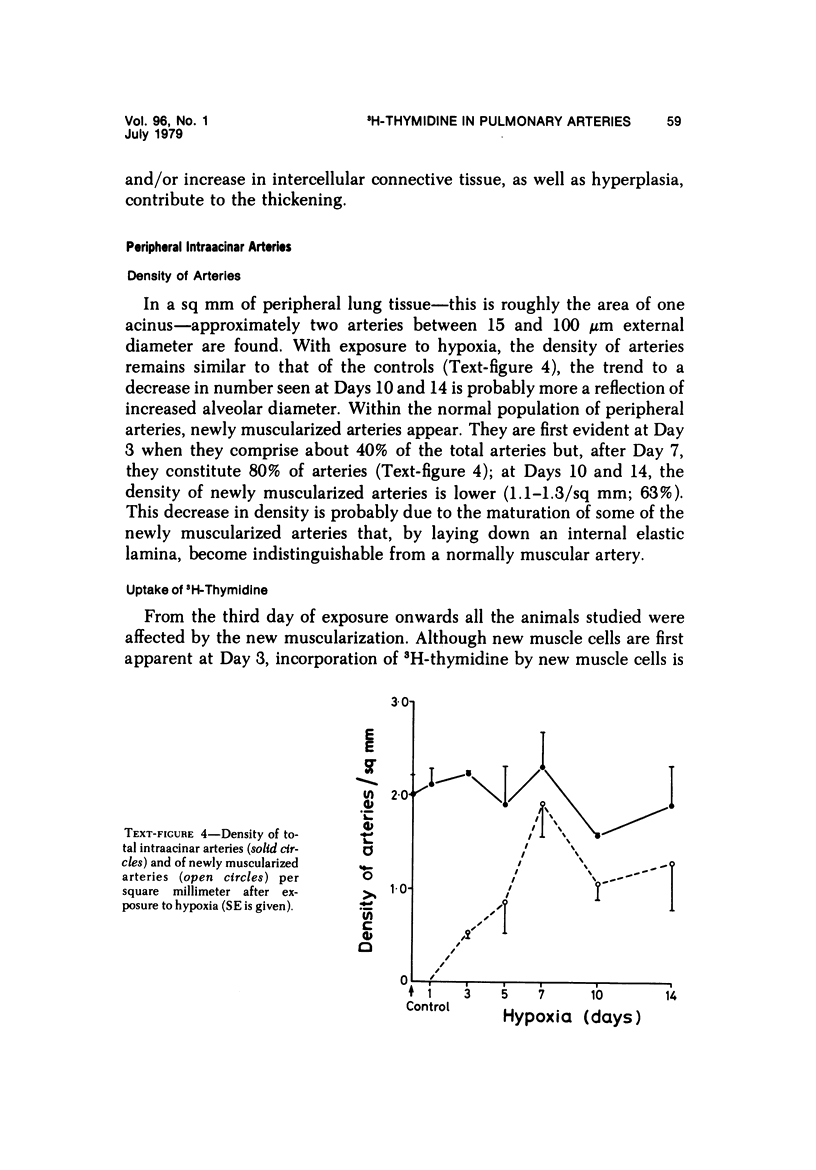
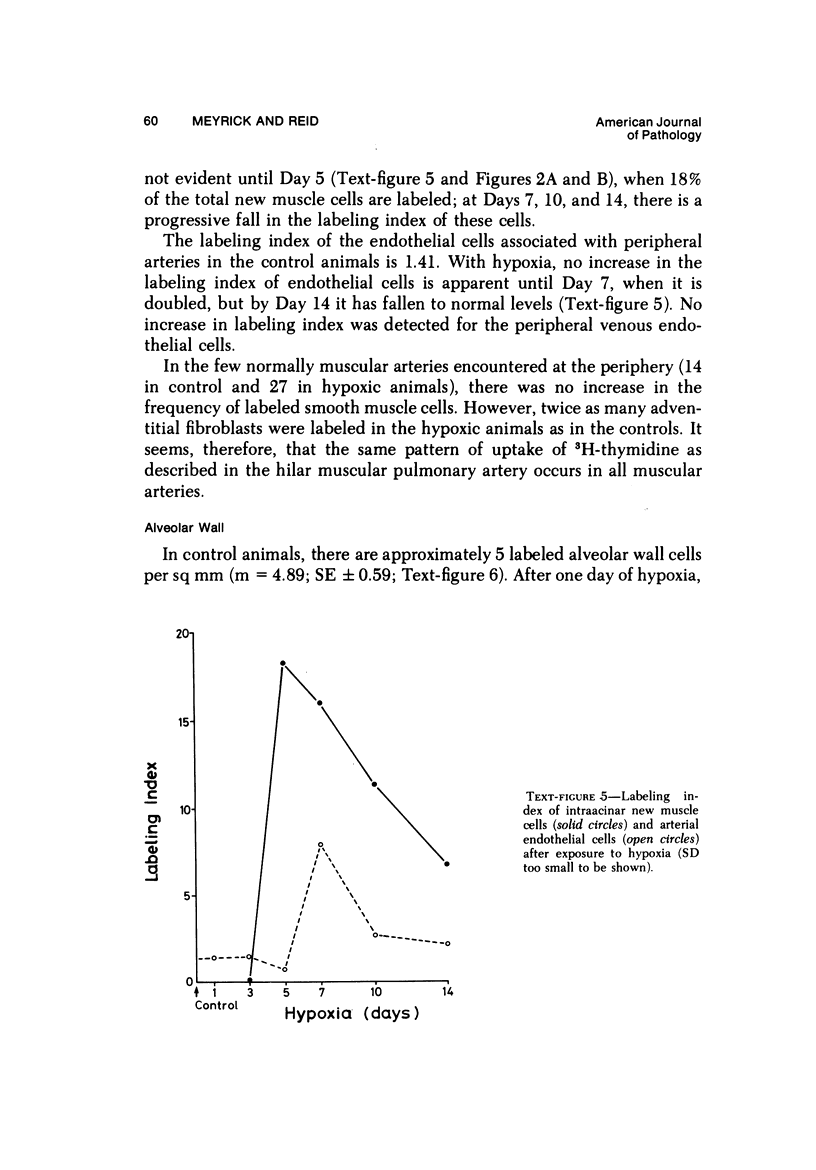
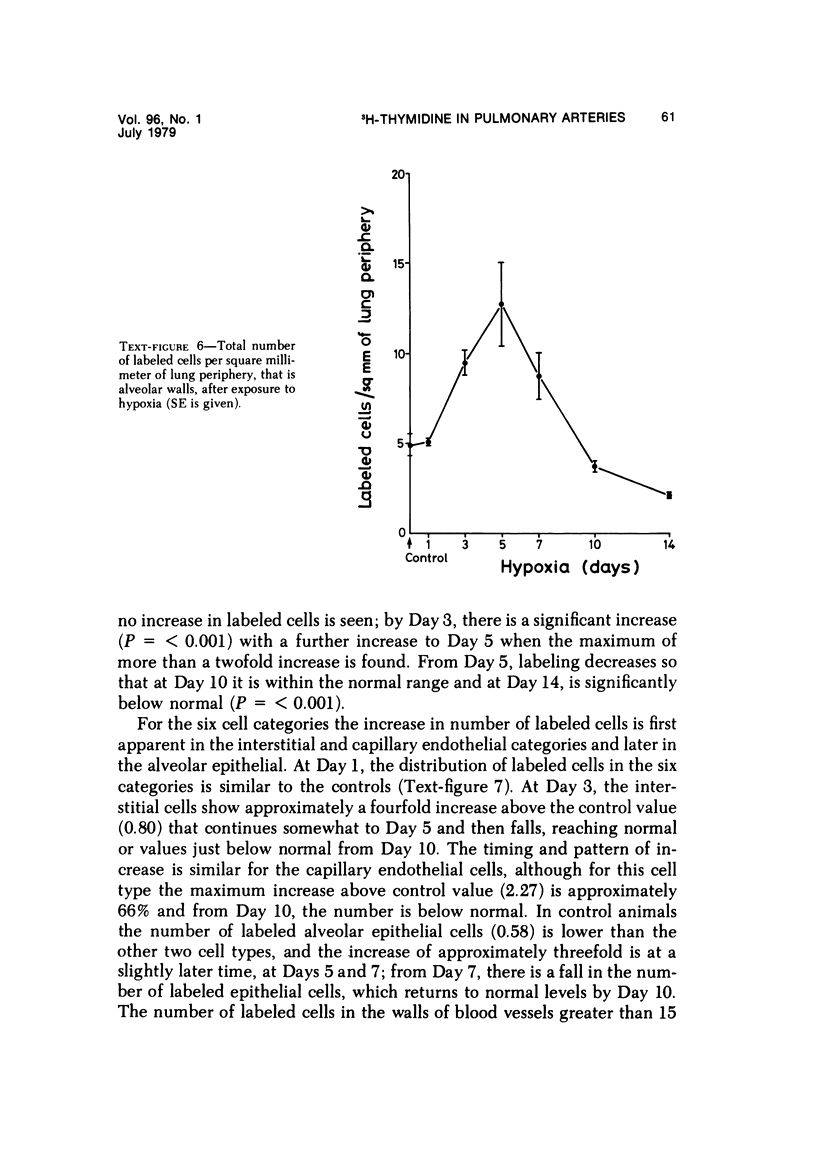
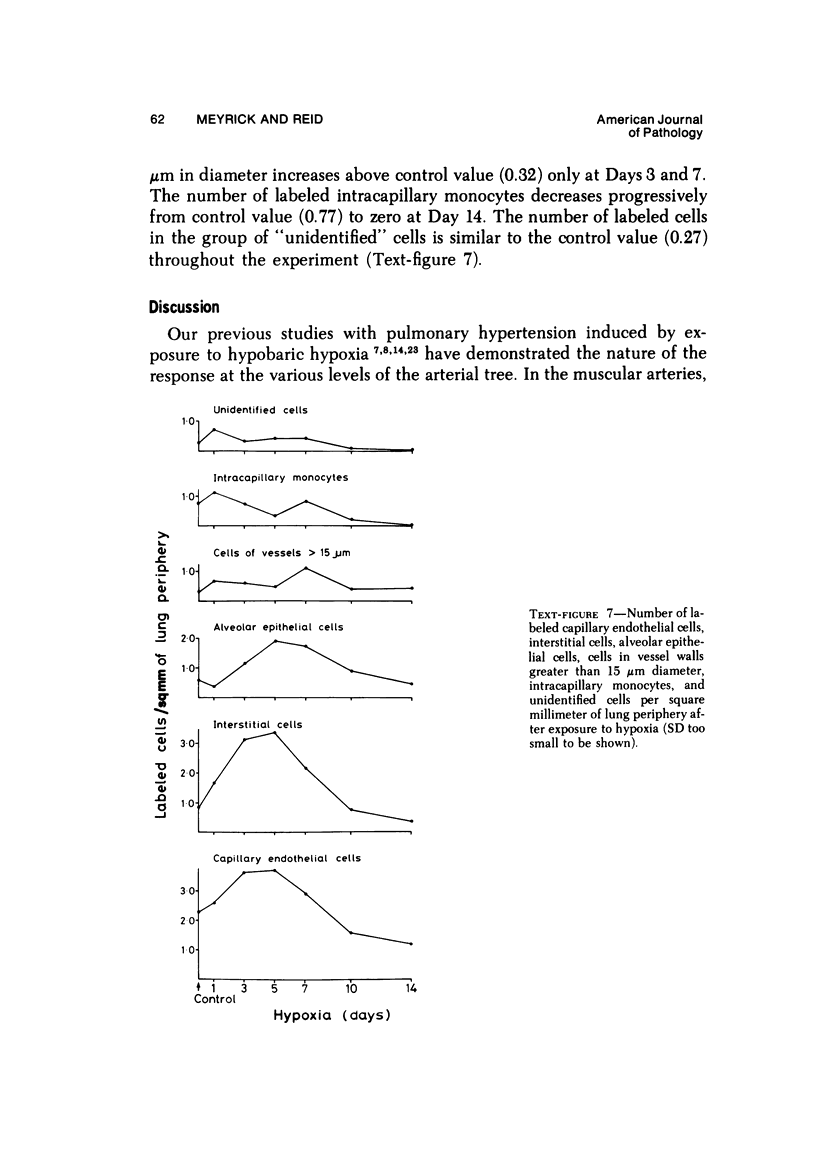
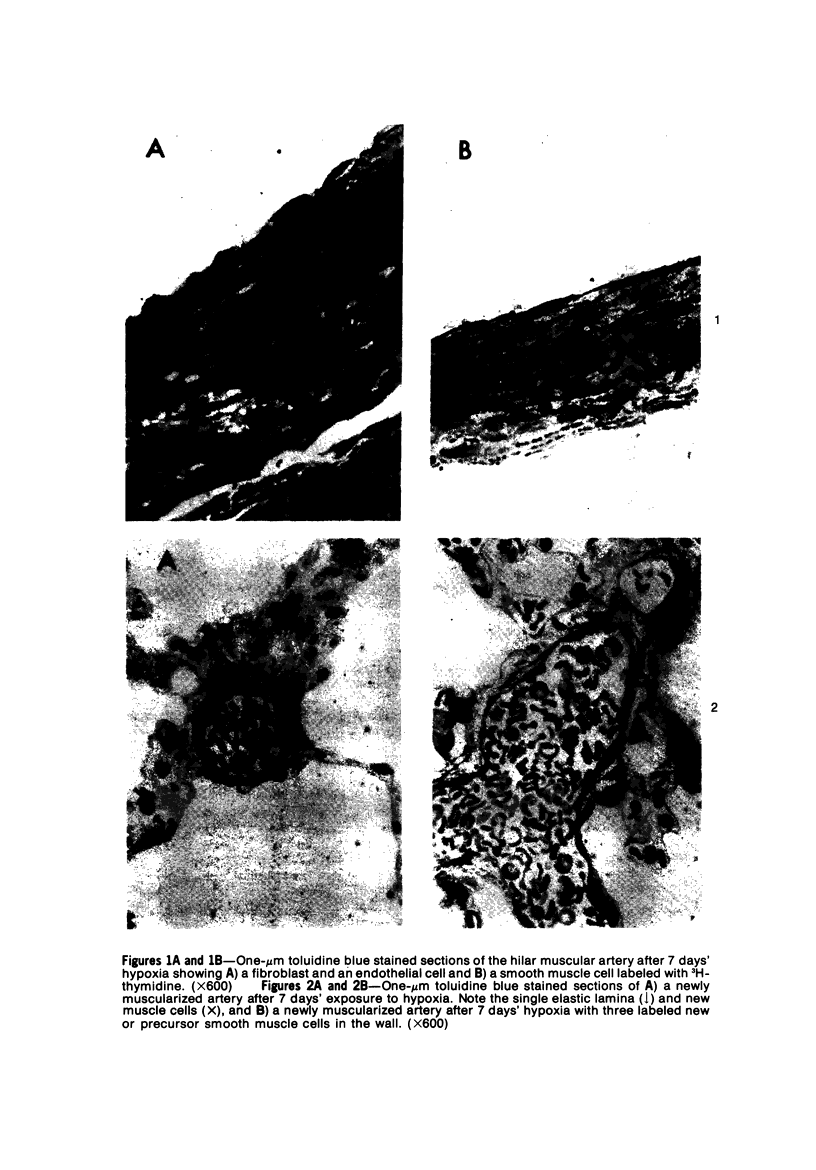
In the pulmonary arterial circulation hypoxia produces increase in thickness of the medial muscle coat as well as of the adventitia; in addition muscle appears in smaller arteries than is normal and the number of small arteries that fill on Micropaque-gelatin injection is reduced. To assess the role of hyperplasia in these changes, the uptake of 3H-thymidine by the cells of the pulmonary arterial wall has been studied in rats exposed to hypobaric hypoxia (exposure to 380 torr) after 1, 3, 5, 7, 10, and 14 days. Using autoradiographs of 1-micron sections, the glutaraldehyde-distended intrapulmonary hilar muscular artery, the peripheral, intraacinar arteries less than 100 micron in external diameter, and the alveolar wall had different patterns of uptake. In the hilar pulmonary artery, after 24 hours of exposure, the labeling index for adventitial fibroblasts is increased eightfold over the control value, and for endothelial cells, threefold, while for medial smooth muscle cells, there is a gradual and small increase to Day 14. Newly muscularized intraacinar arteries are first apparent at Day 3, when they comprise 40% of the intraacinar arteries, increasing to 80% at Day 7. No decrease in density of arteries is found. Uptake of 3H-thymidine by new muscle cells is not apparent until Day 5 when labeling is maximum. The endothelial cells of the newly muscularized arteries show an increased labeling index only at Days 7 and 10. The veins and normally muscular arteries do not show these changes. In the alveolar walls, the concentration of labeled cells is significantly above the control value at Days 3, 5, and 7 and significantly below, at Day 14. At this level, the interstitial, epithelial, and endothelial cells contribute to the increase.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIAS-STELLA J., SALDANA M. THE TERMINAL PORTION OF THE PULMONARY ARTERIAL TREE IN PEOPLE NATIVE TO HIGH ALTITUDES. Circulation. 1963 Nov;28:915–925. doi: 10.1161/01.cir.28.5.915. [DOI] [PubMed] [Google Scholar]
- Abraham A. S., Kay J. M., Cole R. B., Pincock A. C. Haemodynamic and pathological study of the effect of chronic hypoxia and subsequent recovery of the heart and pulmonary vasculature of the rat. Cardiovasc Res. 1971 Jan;5(1):95–102. doi: 10.1093/cvr/5.1.95. [DOI] [PubMed] [Google Scholar]
- Bevan R. D. An autoradiographic and pathological study of cellular proliferation in rabbit arteries correlated with an increase in arterial pressure. Blood Vessels. 1976;13(1-2):100–128. doi: 10.1159/000158083. [DOI] [PubMed] [Google Scholar]
- Burri P. H., Weibel E. R. Morphometric estimation of pulmonary diffusion capacity. II. Effect of Po2 on the growing lung, adaption of the growing rat lung to hypoxia and hyperoxia. Respir Physiol. 1971 Jan;11(2):247–264. doi: 10.1016/0034-5687(71)90028-4. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Cunningham E. L., Brody J. S., Jain B. P. Lung growth induced by hypoxia. J Appl Physiol. 1974 Sep;37(3):362–366. doi: 10.1152/jappl.1974.37.3.362. [DOI] [PubMed] [Google Scholar]
- Esterly J. A., Glagov S., Ferguson D. J. Morphogenesis of intimal obliterative hyperplasia of small arteries in experimental pulmonary hypertension. An ultrastructural study of the role of smooth-muscle cells. Am J Pathol. 1968 Feb;52(2):325–347. [PMC free article] [PubMed] [Google Scholar]
- FULTON R. M., HUTCHINSON E. C., JONES A. M. Ventricular weight in cardiac hypertrophy. Br Heart J. 1952 Jul;14(3):413–420. doi: 10.1136/hrt.14.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A., Weibel E. R. Morphometrische Untersuchung der Verteilung einer spezifischen cytoplasmatischen Organelle in Endothelzellen der Ratte. Z Zellforsch Mikrosk Anat. 1966;73(1):1–9. [PubMed] [Google Scholar]
- Hasleton P. S., Heath D., Brewer D. B. Hypertensive pulmonary vascular disease in states of chronic hypoxia. J Pathol Bacteriol. 1968 Apr;95(2):431–440. doi: 10.1002/path.1700950213. [DOI] [PubMed] [Google Scholar]
- Haworth S. G., Reid L. A morphometric study of regional variation in lung structure in infants with pulmonary hypertension and congenital cardiac defect. A justification of lung biopsy. Br Heart J. 1978 Aug;40(8):825–831. doi: 10.1136/hrt.40.8.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D., Edwards C., Winson M., Smith P. Effects on the right ventricle, pulmonary vasculature, and carotid bodies of the rat of exposure to, and recovery from, simulated high altitude. Thorax. 1973 Jan;28(1):24–28. doi: 10.1136/thx.28.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicken P., Heath D., Brewer D. B., Whitaker W. The small pulmonary arteries in emphysema. J Pathol Bacteriol. 1965 Jul;90(1):107–114. doi: 10.1002/path.1700900111. [DOI] [PubMed] [Google Scholar]
- Hislop A., Reid L. Changes in the pulmonary arteries of the rat during recovery from hypoxia-induced pulmonary hypertension. Br J Exp Pathol. 1977 Dec;58(6):653–662. [PMC free article] [PubMed] [Google Scholar]
- Hislop A., Reid L. New findings in pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. Br J Exp Pathol. 1976 Oct;57(5):542–554. [PMC free article] [PubMed] [Google Scholar]
- Hunter C., Barer G. R., Shaw J. W., Clegg E. J. Growth of the heart and lungs in hypoxic rodents: a model of human hypoxic disease. Clin Sci Mol Med. 1974 Mar;46(3):375–391. doi: 10.1042/cs0460375. [DOI] [PubMed] [Google Scholar]
- Jaenke R. S., Alexander A. F. Fine structural alterations of bovine peripheral pulmonary arteries in hypoxia-induced hypertension. Am J Pathol. 1973 Nov;73(2):377–398. [PMC free article] [PubMed] [Google Scholar]
- Kapanci Y., Weibel E. R., Kaplan H. P., Robinson F. R. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. II. Ultrastructural and morphometric studies. Lab Invest. 1969 Jan;20(1):101–118. [PubMed] [Google Scholar]
- Meyrick B., Hislop A., Reid L. Pulmonary arteries of the normal rat: the thick walled oblique muscle segment. J Anat. 1978 Feb;125(Pt 2):209–221. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Miller J., Reid L. Pulmonary oedema induced by ANTU, or by high or low oxygen concentrations in rat--an electron microscopic study. Br J Exp Pathol. 1972 Aug;53(4):347–358. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Reid L. Development of pulmonary arterial changes in rats fed Crotalaria spectabilis. Am J Pathol. 1979 Jan;94(1):37–50. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab Invest. 1978 Feb;38(2):188–200. [PubMed] [Google Scholar]
- Meyrick B., Reid L. Ultrastructural features of the distended pulmonary arteries of the normal rat. Anat Rec. 1979 Jan;193(1):71–97. doi: 10.1002/ar.1091930106. [DOI] [PubMed] [Google Scholar]
- NAEYE R. L. Hypoxemia and pulmonary hypertension. A study of the pulmonary vasculature. Arch Pathol. 1961 Apr;71:447–452. [PubMed] [Google Scholar]
- Novi A. M. Molecular basis of a control mechanism of DNA synthesis in mammalian cells. Klin Wochenschr. 1976 Oct 15;54(20):961–968. doi: 10.1007/BF01468946. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., Haworth S. G., Castaneda A. R., Nadas A. S., Reid L. M. Lung biopsy in congenital heart disease: a morphometric approach to pulmonary vascular disease. Circulation. 1978 Dec;58(6):1107–1122. doi: 10.1161/01.cir.58.6.1107. [DOI] [PubMed] [Google Scholar]
- Ryland D., Reid L. The pulmonary circulation in cystic fibrosis. Thorax. 1975 Jun;30(3):285–292. doi: 10.1136/thx.30.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmens M., Reid L. Pulmonary arterial muscularity and right ventricular hypertrophy in chronic bronchitis and emphysema. Br J Dis Chest. 1974 Oct;68:253–263. doi: 10.1016/0007-0971(74)90049-7. [DOI] [PubMed] [Google Scholar]
- Shelton D. M., Keal E., Reid L. The pulmonary circulation in chronic bronchitis and emphysema. Chest. 1977 Feb;71(2 Suppl):303–306. doi: 10.1378/chest.71.2_supplement.303. [DOI] [PubMed] [Google Scholar]
- Smith P., Moosavi H., Winson M., Heath D. The influence of age and sex on the response of the right ventricle, pulmonary vasculature and carotid bodies to hypoxia in rats. J Pathol. 1974 Jan;112(1):11–18. doi: 10.1002/path.1711120104. [DOI] [PubMed] [Google Scholar]
- Stephens R. J., Sloan M. F., Evans M. J., Freeman G. Early response of lung to low levels of ozone. Am J Pathol. 1974 Jan;74(1):31–58. [PMC free article] [PubMed] [Google Scholar]
- Völkel N., Wiegers U., Sill V., Trautmann J. A kinetic study of lung DNA-synthesis during simulated chronic high altitude hypoxia. Thorax. 1977 Oct;32(5):578–581. doi: 10.1136/thx.32.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRIGHT B. M. APPARATUS FOR EXPOSING ANIMALS TO REDUCED ATMOSPHERIC PRESSURE FOR LONG PERIODS. Br J Haematol. 1964 Jan;10:75–77. doi: 10.1111/j.1365-2141.1964.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Yuen T. G., Sherwin R. P. Hyperplasia of type 2 pneumocytes and nitrogen dioxide (10 ppm) exposure. A quantitation based on electron photomicrographs. Arch Environ Health. 1971 Jan;22(1):178–188. doi: 10.1080/00039896.1971.10665830. [DOI] [PubMed] [Google Scholar]