Abstract
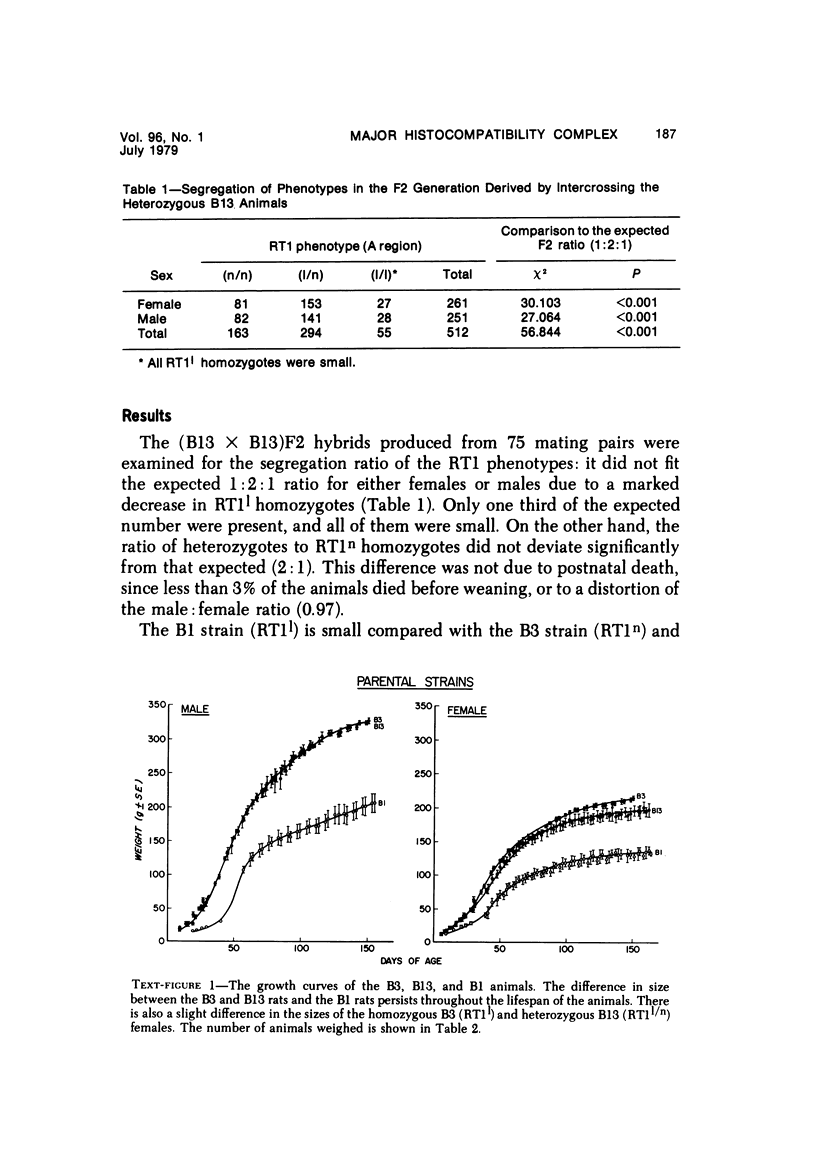
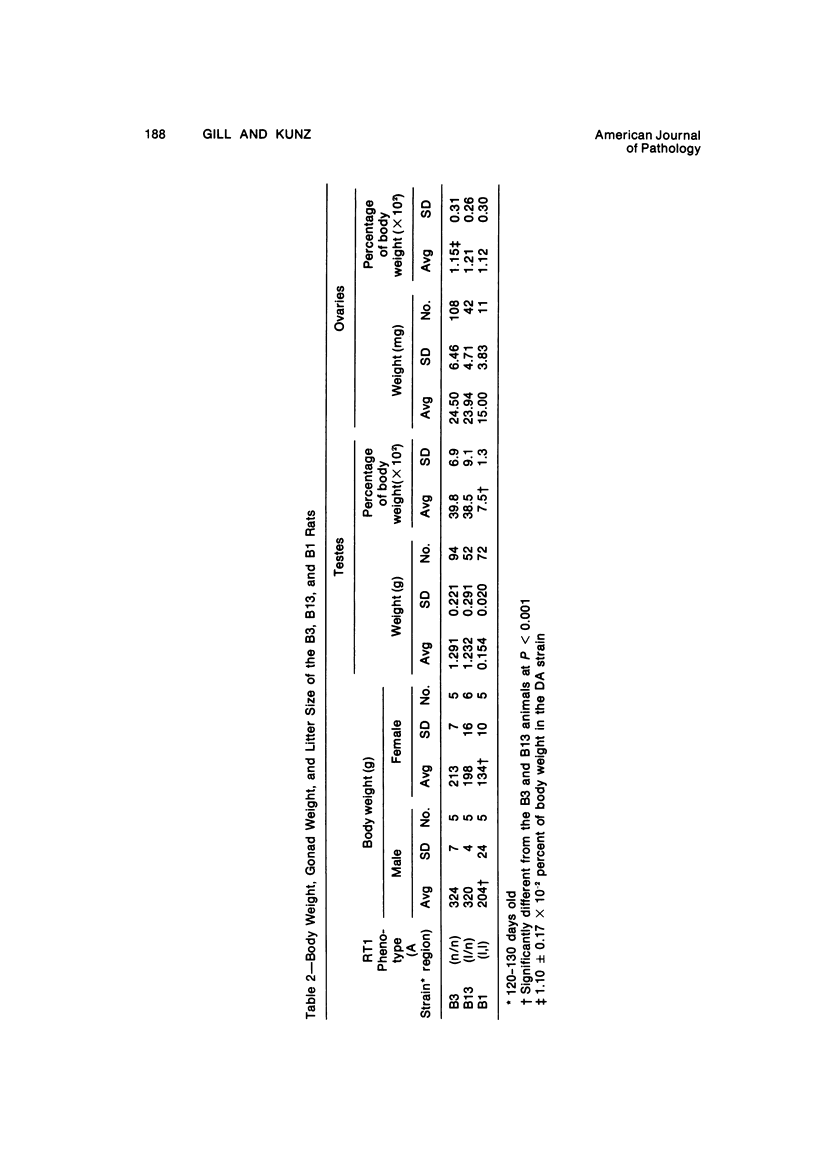
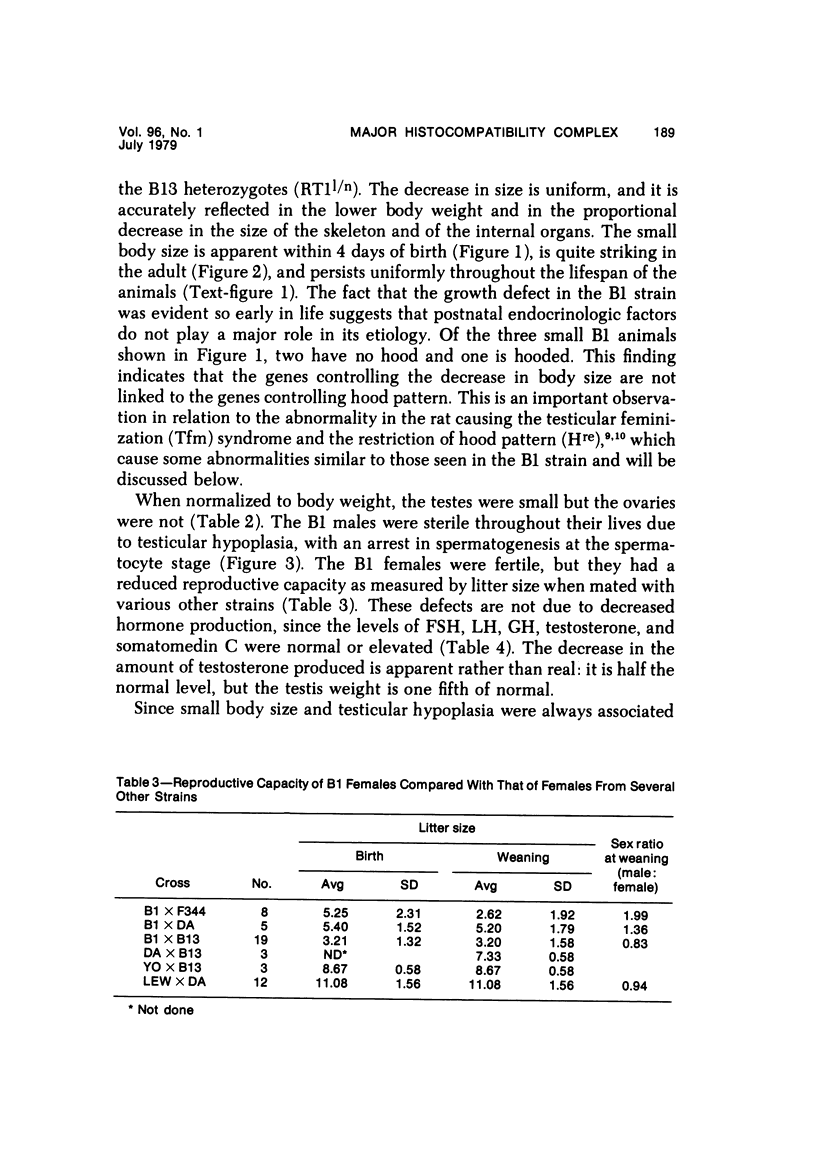
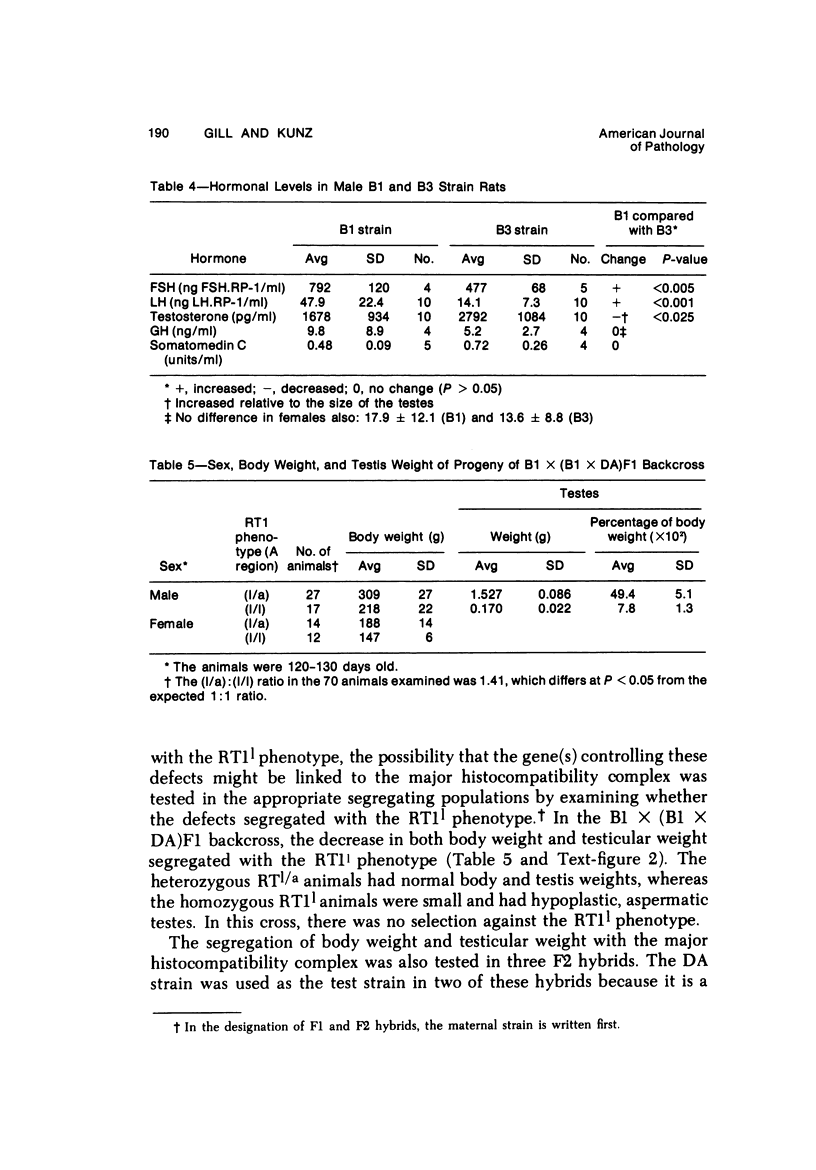
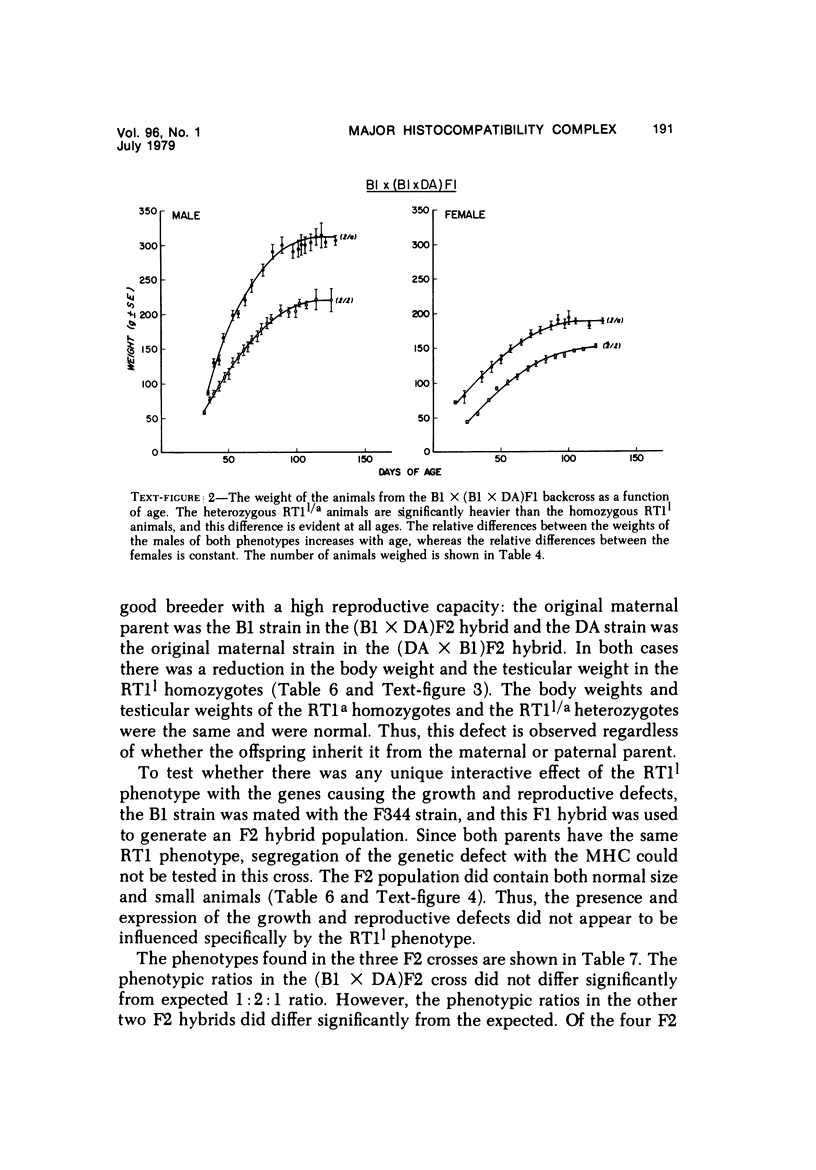
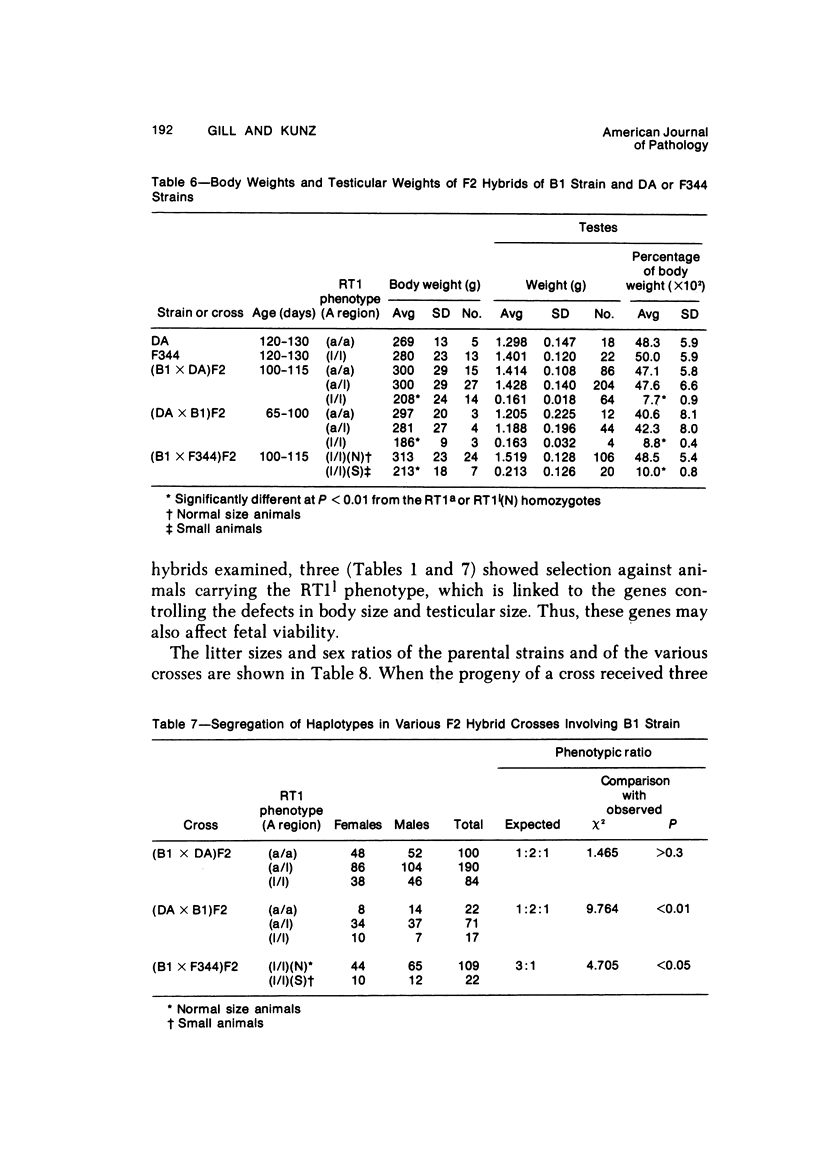
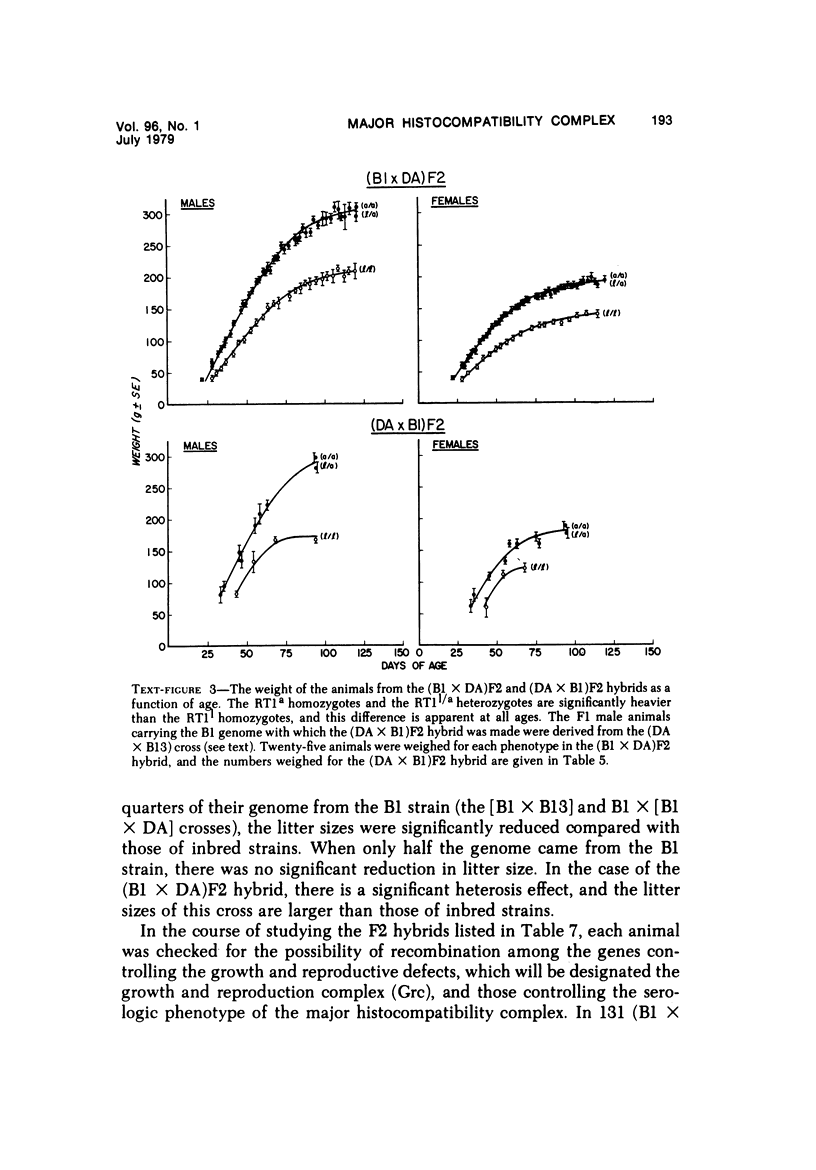
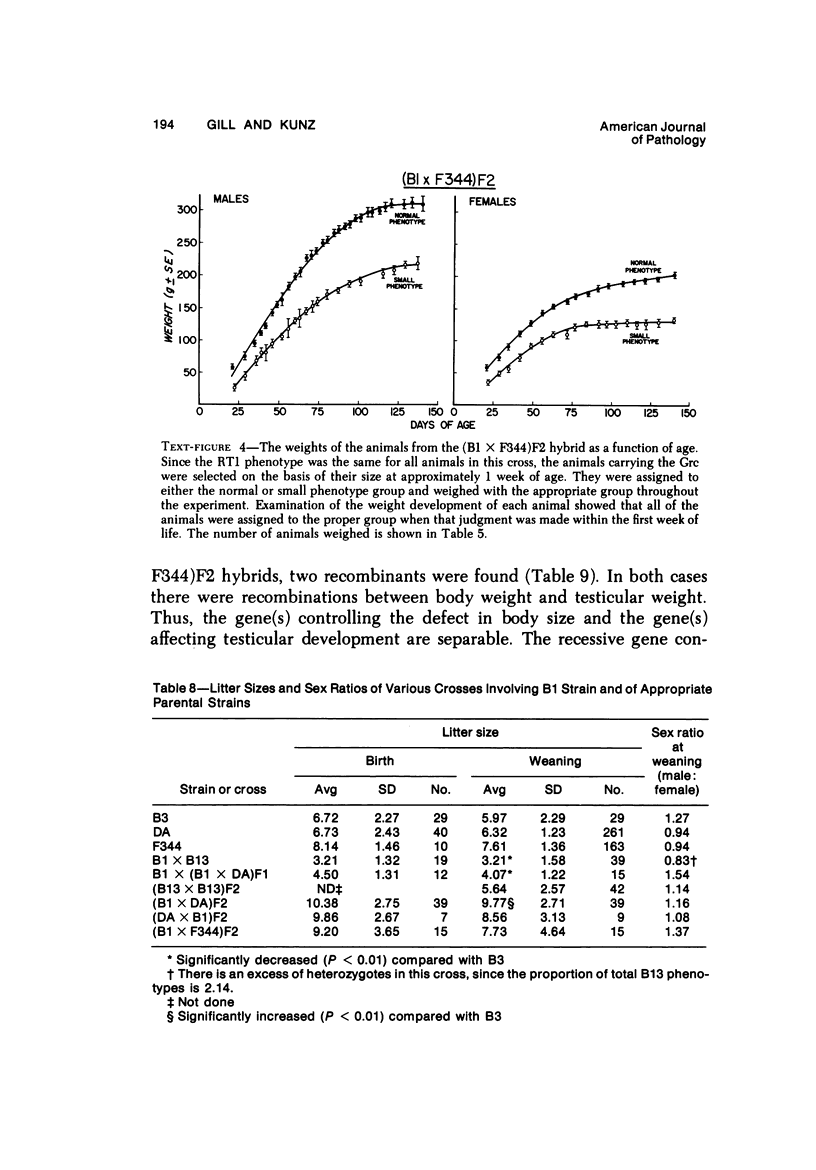
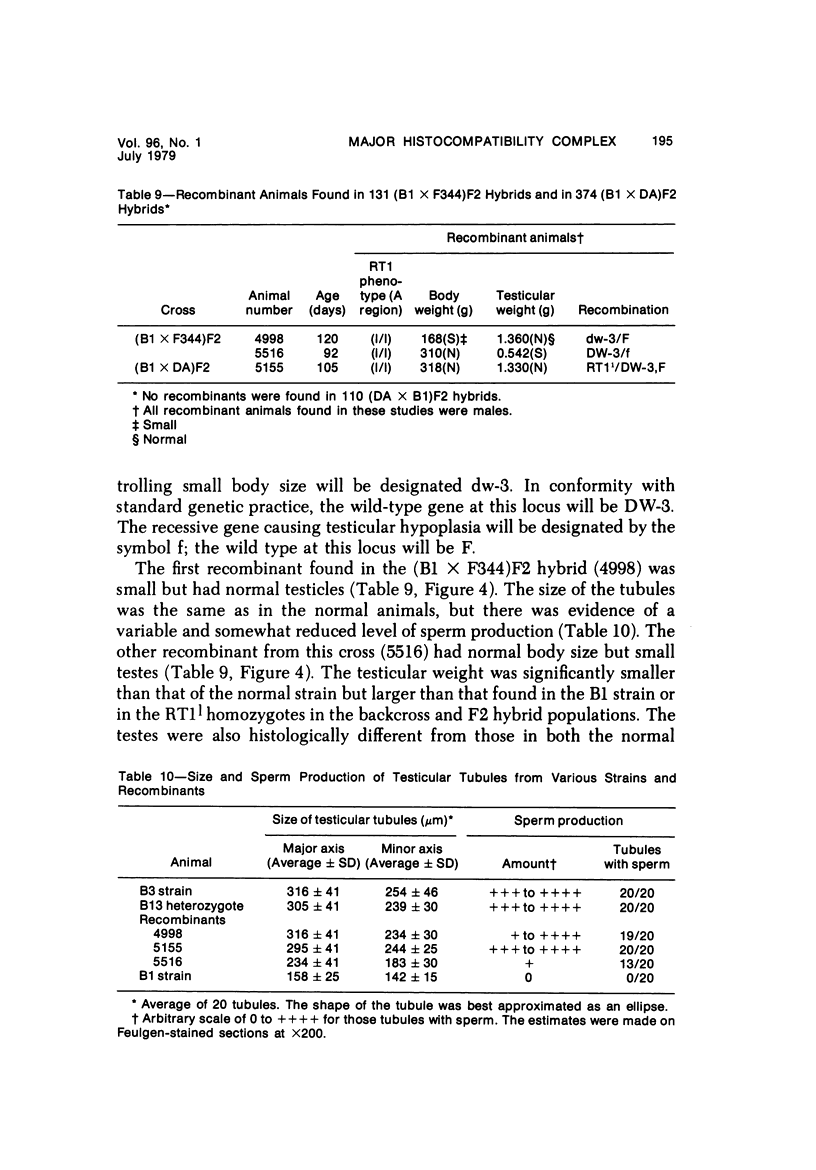
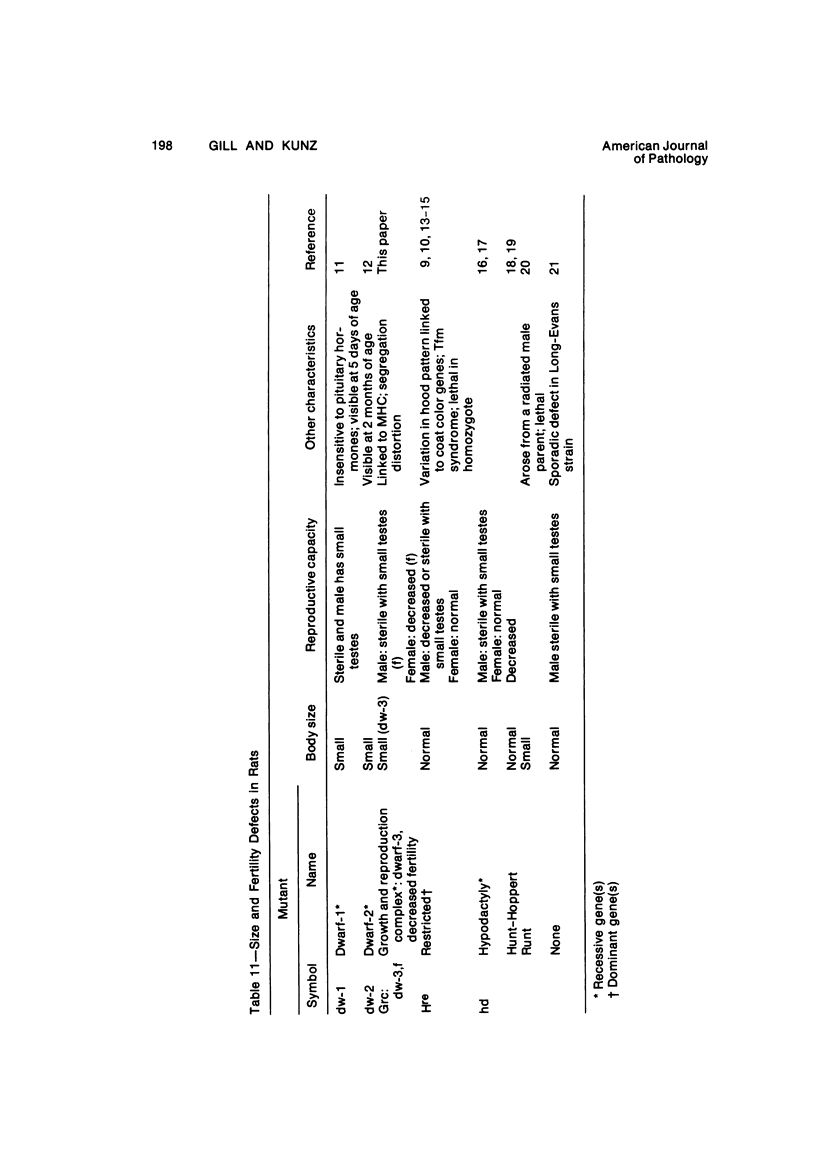
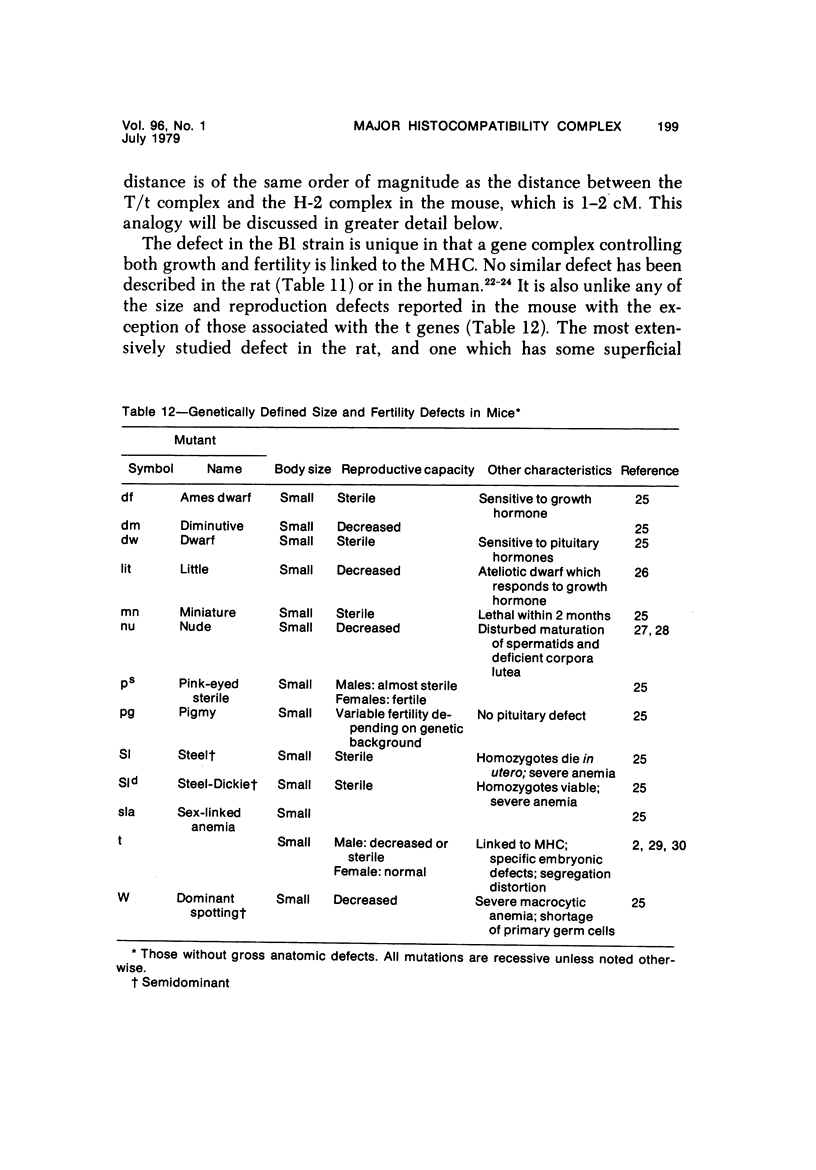
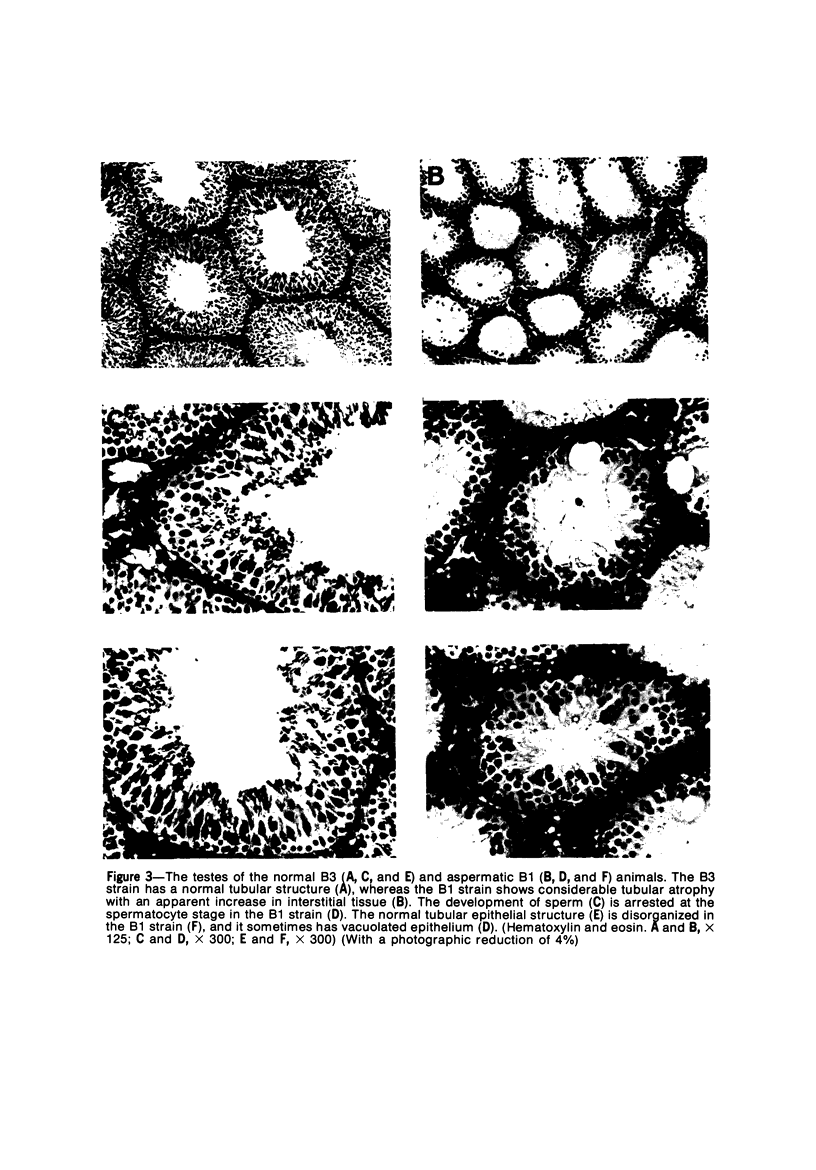
The B1 strain of rats carries a unique mutation which causes defects in growth and reproduction: the males and females are small, the testes are hypoplastic and aspermatic, and the females have a reduced reproductive capacity. The loci controlling these defects are linked to the major histocompatibility complex (MHC) as determined by segregation studies in backcross and F2 hybrid populations. The levels of pituitary hormones and somatomedin C in the B1 strain are elevated or normal, and the testosterone level is elevated relative to the size of the testes. These findings suggest that hormone deficiencies are not the cause of these defects. The genes governing these defects have been designated the growth and reproduction complex (Grc). The recessive gene regulating small body size has been designated dw-3 (dwarf-3), and the recessive gene influencing reproductive capacity has been designated f. The Grc and MHC are separable by recombination, and the dw-3 and f genes are also separable by recombination. Studies in the (B1 X DA)F2 hybrid indicate that the map distance between the Grc and the MHC is 0.6 cM. Segregation distortion due to a deficiency of RT11 homozygotes is seen in some F2 hybrid populations derived from the B1 strain. Litter size data suggest that the loss of the RT11 homozygotes is due to intrauterine death. There is no apparent sex influence on the inheritance of the Grc, at least as it is presently understood, since it can be transmitted by either females or males. The growth and reproduction complex in the rat may be the analog of the T/t complex in the mouse, and the importance of the region of the chromosome adjacent to the major histocompatibility complex in the control of developmental processes may be a general phenomenon in mammals.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardin C. W., Bullock L. P., Sherins R. J., Mowszowicz I., Blackburn W. R. Androgen metabolism and mechanism of action in male pseudohermaphroditism: a study of testicular feminization. Recent Prog Horm Res. 1973;29:65–109. doi: 10.1016/b978-0-12-571129-6.50006-3. [DOI] [PubMed] [Google Scholar]
- Bobrow M., Bodmer J. G., Bodmer W. F., McDevitt H. O., Lorber J., Swift P. The search for a human equivalent of the mouse T-locus - negative results from a study of HL-A types in spina bifida. Tissue Antigens. 1975 May;5(4):234–237. doi: 10.1111/j.1399-0039.1975.tb01469.x. [DOI] [PubMed] [Google Scholar]
- De Bruyere M., Kulakowski S., Malchaire J., Delire M., Sokal G. HLA gene and haplotype frequencies in spina bifida. Population and family studies. Tissue Antigens. 1977 Nov;10(5):399–402. doi: 10.1111/j.1399-0039.1977.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Beamer W. G. Inherited ateliotic dwarfism in mice. Characteristics of the mutation, little, on chromosome 6. J Hered. 1976 Mar-Apr;67(2):87–91. doi: 10.1093/oxfordjournals.jhered.a108682. [DOI] [PubMed] [Google Scholar]
- Flanagan S. P. 'Nude', a new hairless gene with pleiotropic effects in the mouse. Genet Res. 1966 Dec;8(3):295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- Furlanetto R. W., Underwood L. E., Van Wyk J. J., D'Ercole A. J. Estimation of somatomedin-C levels in normals and patients with pituitary disease by radioimmunoassay. J Clin Invest. 1977 Sep;60(3):648–657. doi: 10.1172/JCI108816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T. J., 3rd, Cramer D. V., Kunz H. W. The major histocompatibility complex--comparison in the mouse, man, and the rat. A review. Am J Pathol. 1978 Mar;90(3):737–778. [PMC free article] [PubMed] [Google Scholar]
- Goodman R. L., Hotchkiss J., Karsch F. J., Knobil E. Diurnal variations in serum testosterone concentrations in the adult male rhesus monkey. Biol Reprod. 1974 Dec;11(5):624–630. doi: 10.1095/biolreprod11.5.624. [DOI] [PubMed] [Google Scholar]
- Gumbreck L. G., Stanley A. J., Allison J. E., Easley R. B. Restriction of color in the rat with associated sterility in the male and heterochromia in both sexes. J Exp Zool. 1972 Jun;180(3):333–350. doi: 10.1002/jez.1401800305. [DOI] [PubMed] [Google Scholar]
- Gumbreck L. G., Stanley A. J., Macy R. M., Peeples E. E. Pleiotropic expression of the restricted coat-color gene in the Norway rat. J Hered. 1971 Nov-Dec;62(6):356–358. [PubMed] [Google Scholar]
- Klein J., Hammerberg C. The control of differentiation by the T complex. Immunol Rev. 1977 Jan;33:70–104. doi: 10.1111/j.1600-065x.1977.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Martin J. B., Harris C. A., Dirks J. H. Evidence of GH deficiency in the Munich-Wistar rat. Endocrinology. 1974 May;94(5):1359–1363. doi: 10.1210/endo-94-5-1359. [DOI] [PubMed] [Google Scholar]
- Naess O., Haug E., Attramadal A., Aakvaag A., Hansson V., French F. Androgen receptors in the anterior pituitary and central nervous system of the androgen "insensitive" (Tfm) rat: correlation between receptor binding and effects of androgens on gonadotropin secretion. Endocrinology. 1976 Nov;99(5):1295–1303. doi: 10.1210/endo-99-5-1295. [DOI] [PubMed] [Google Scholar]
- Niswender G. D., Midgley A. R., Jr, Monroe S. E., Reichert L. E., Jr Radioimmunoassay for rat luteinizing hormone with antiovine LH serum and ovine LH-131-I. Proc Soc Exp Biol Med. 1968 Jul;128(3):807–811. doi: 10.3181/00379727-128-33129. [DOI] [PubMed] [Google Scholar]
- ROSEN S., SREEBNY L. M., HOPPERT C. A., HUNT H. R., BACHEM E. Studies on salivariadenectomized Hunt-Hoppert caries-resistant and Osborne-Mendel-susceptible (OMS) rats. IV. Effect of selective salivariadenectomy on dental caries and several groups of the oral microflora. J Dent Res. 1959 Nov-Dec;38:1166–1172. doi: 10.1177/00220345590380061501. [DOI] [PubMed] [Google Scholar]
- Royer M., Bérard J. J., Toyama K. Anomalies de la spermatogenèse chez un rat mutant. C R Acad Sci Hebd Seances Acad Sci D. 1974 Apr 17;278(16):2045–2047. [PubMed] [Google Scholar]
- Russell L. D., Gardner P. J. Testicular ultrastructure and fertility in the restricted color (H-re) rat. Biol Reprod. 1974 Dec;11(5):631–643. doi: 10.1095/biolreprod11.5.631. [DOI] [PubMed] [Google Scholar]
- SREEBNY L. M., ROSEN S., BACHEM E., HUNT H. R., HOPPERT C. A. The effect of castration on the submaxillary gland of Hunt-Hoppert caries-resistant and caries-susceptible rats. J Dent Res. 1959 Jan-Feb;38(1):67–71. doi: 10.1177/00220345590380012501. [DOI] [PubMed] [Google Scholar]
- Shire J. G., Pantelouris E. M. Comparison of endocrine function in normal and genetically athymic mice. Comp Biochem Physiol A Comp Physiol. 1974 Jan;47(1):93–100. doi: 10.1016/0300-9629(74)90055-3. [DOI] [PubMed] [Google Scholar]
- Snell G. D. The H-2 locus of the mouse: observations and speculations concerning its comparative genetics and its polymorphism. Folia Biol (Praha) 1968;14(5):335–358. [PubMed] [Google Scholar]
- Taylor B. A. The frequency of x-ray induced recessive visible mutations in the rat. Genetics. 1968 Nov;60(3):559–565. doi: 10.1093/genetics/60.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]