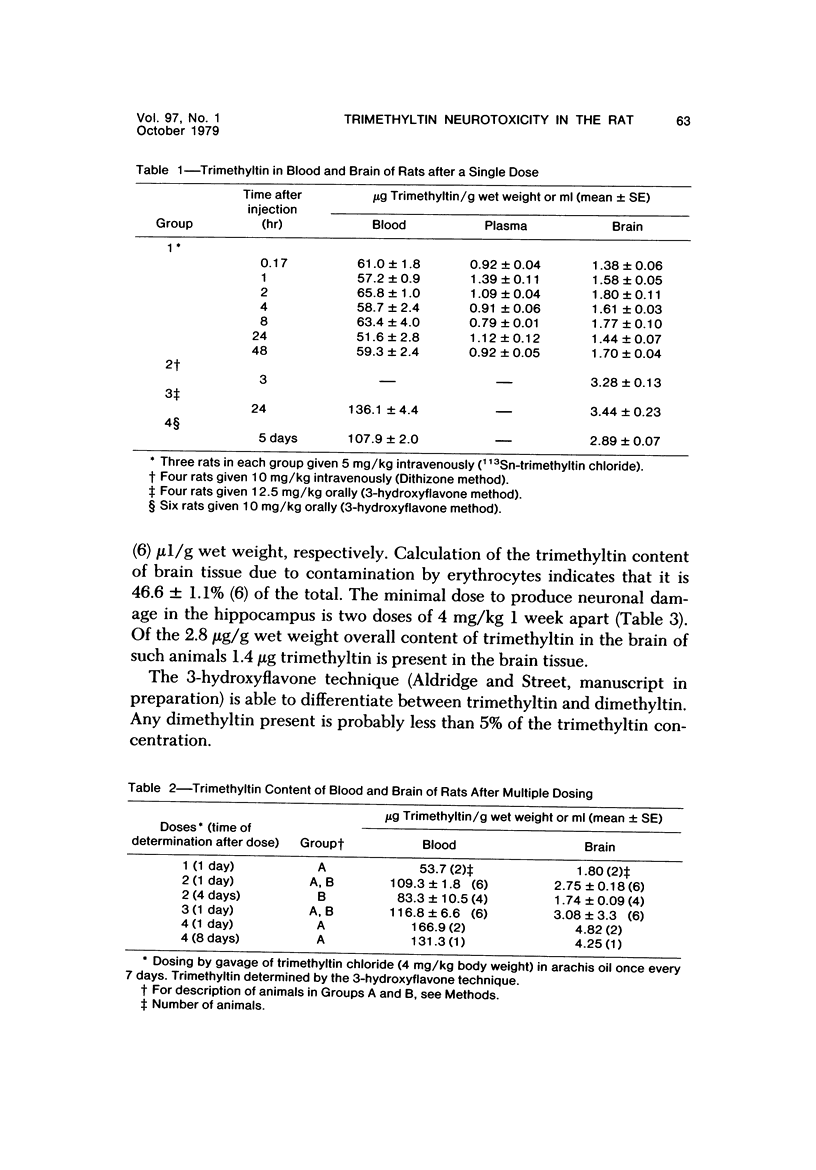
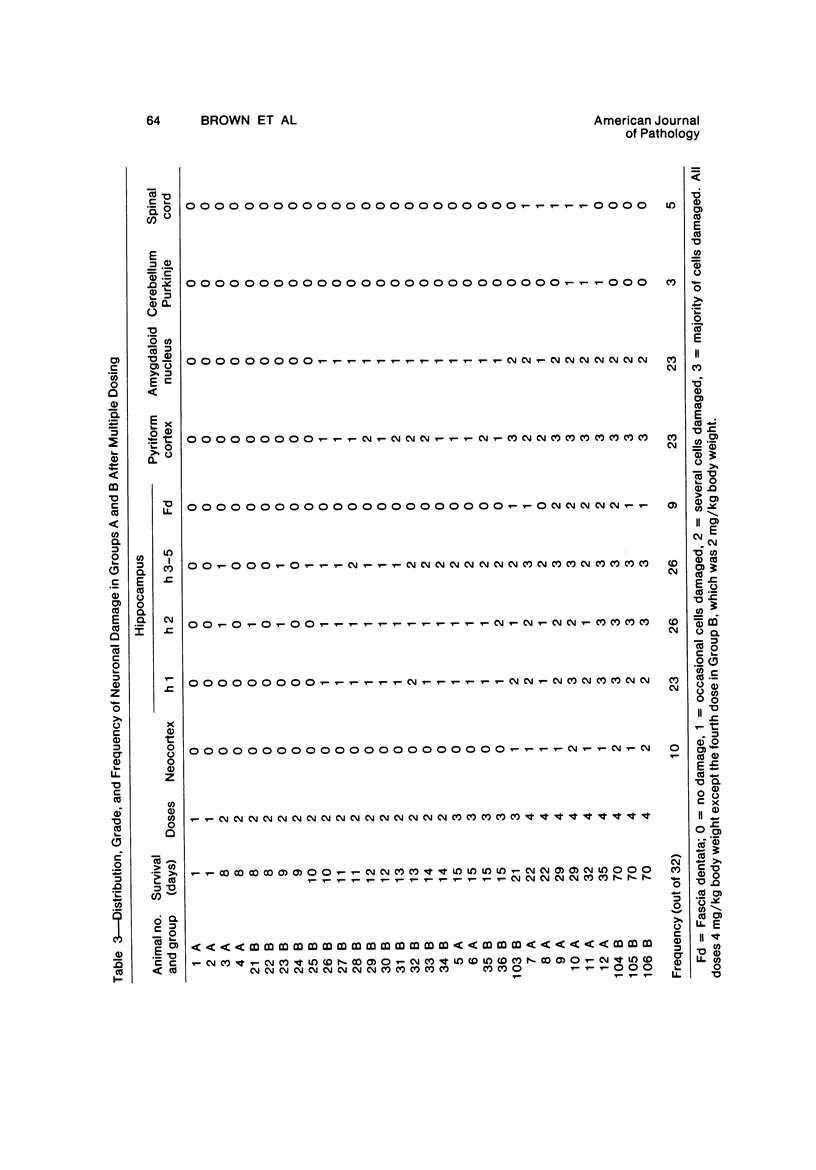
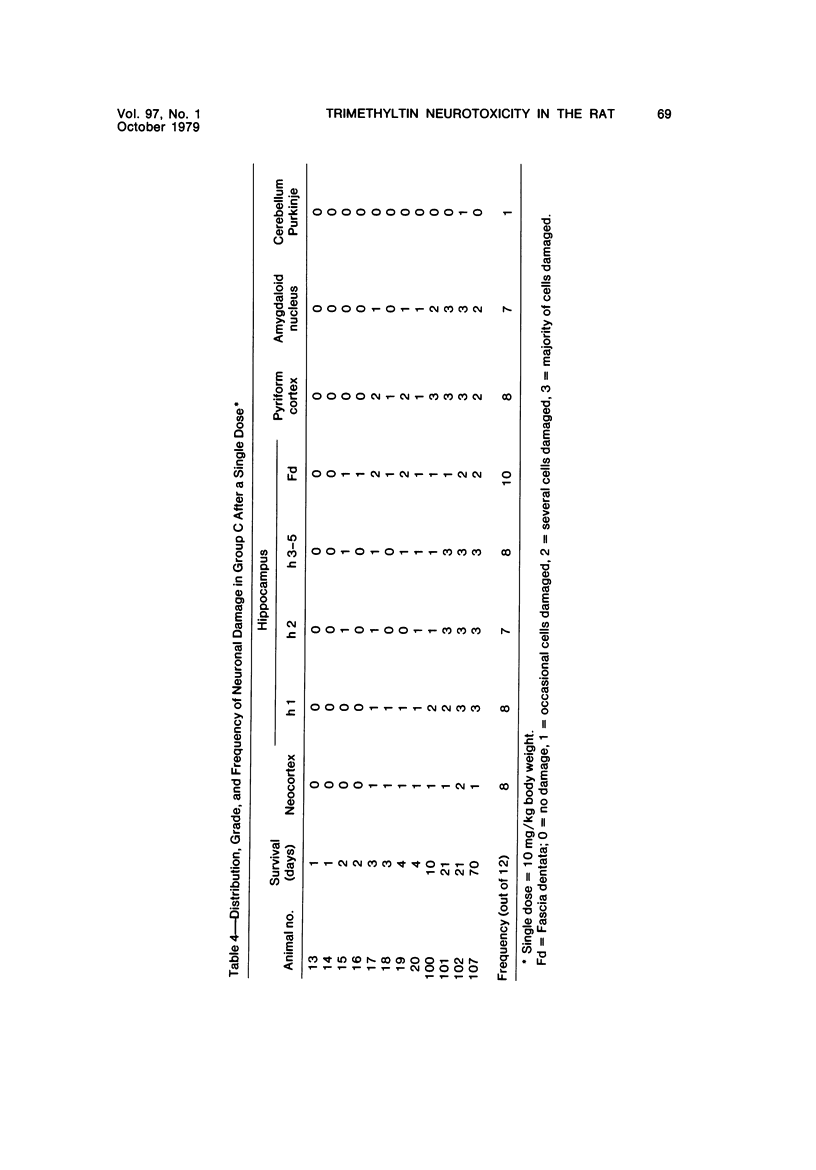
Abstract
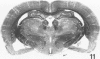
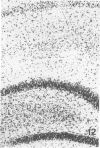
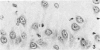
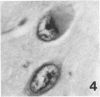
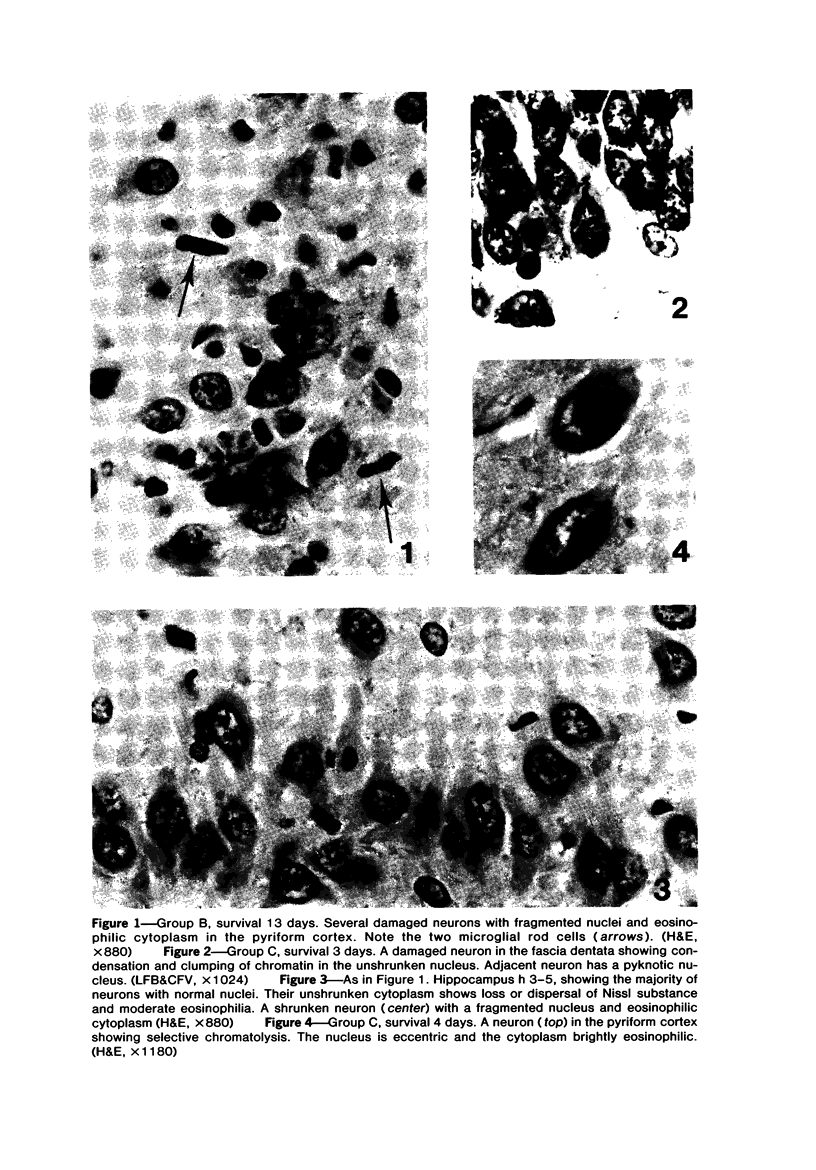
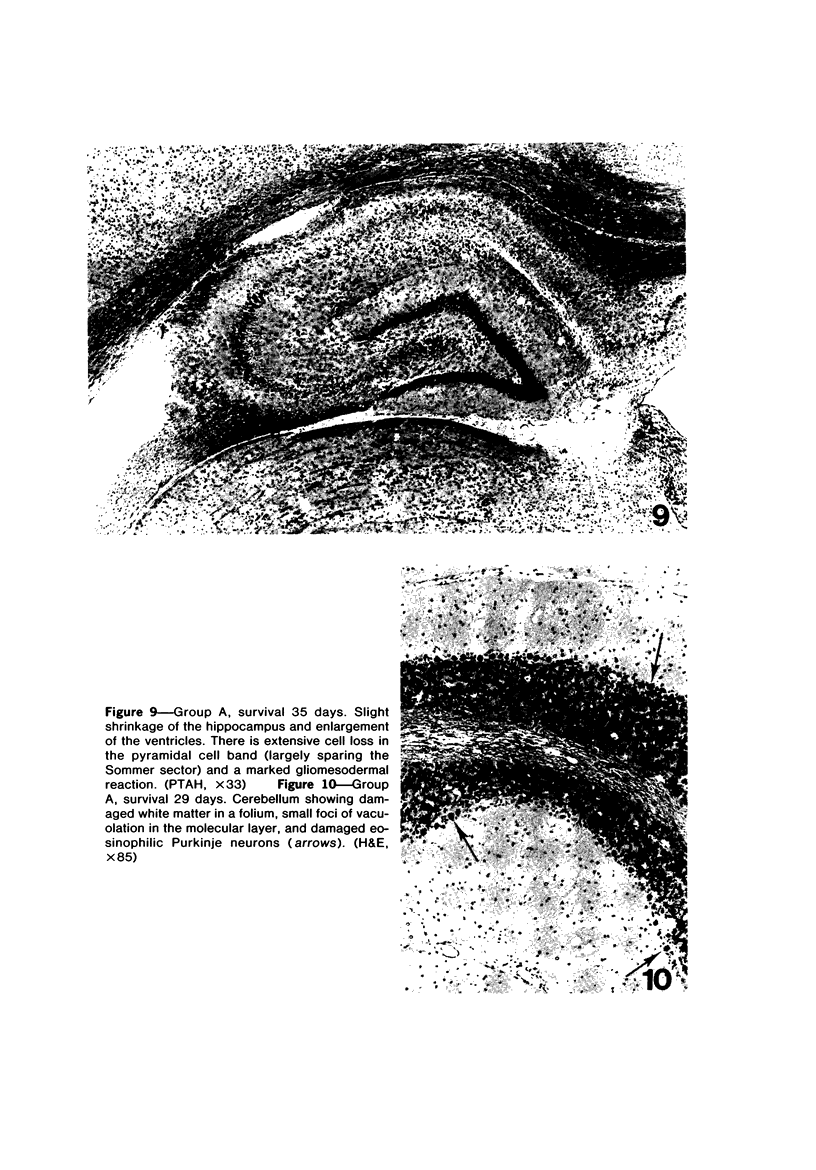
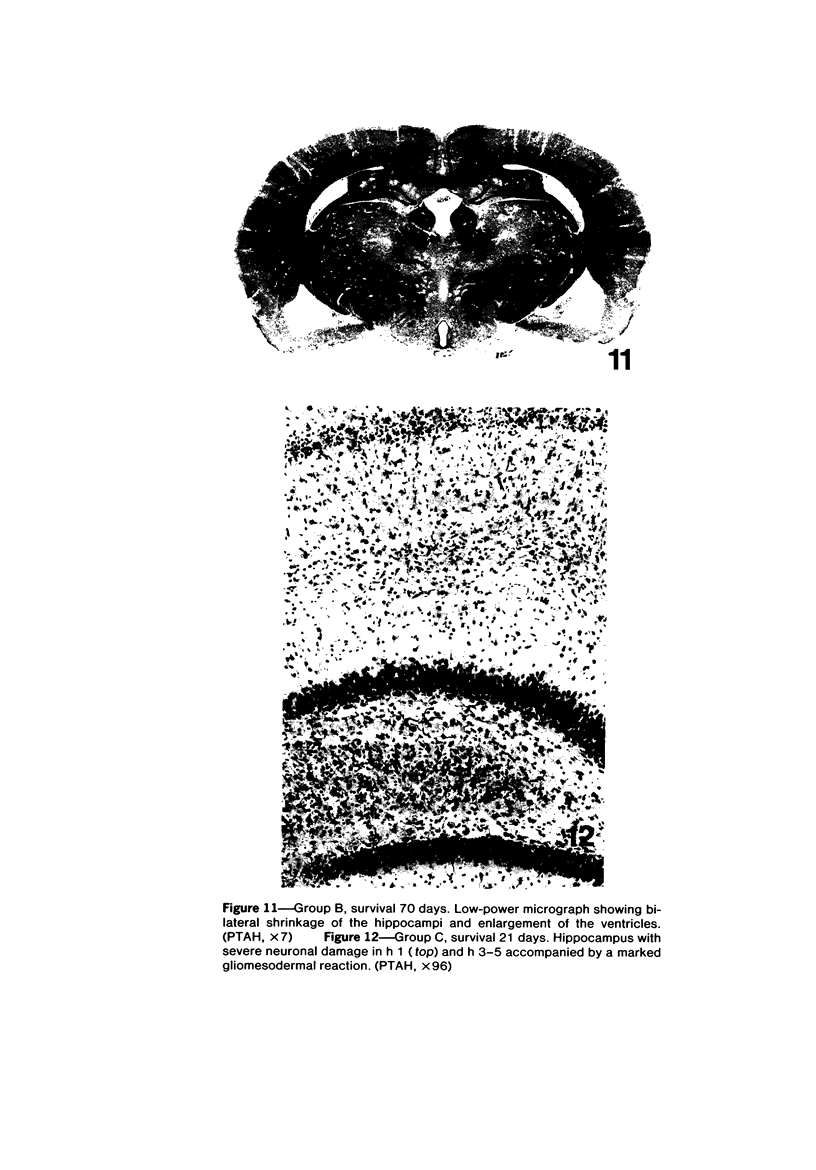
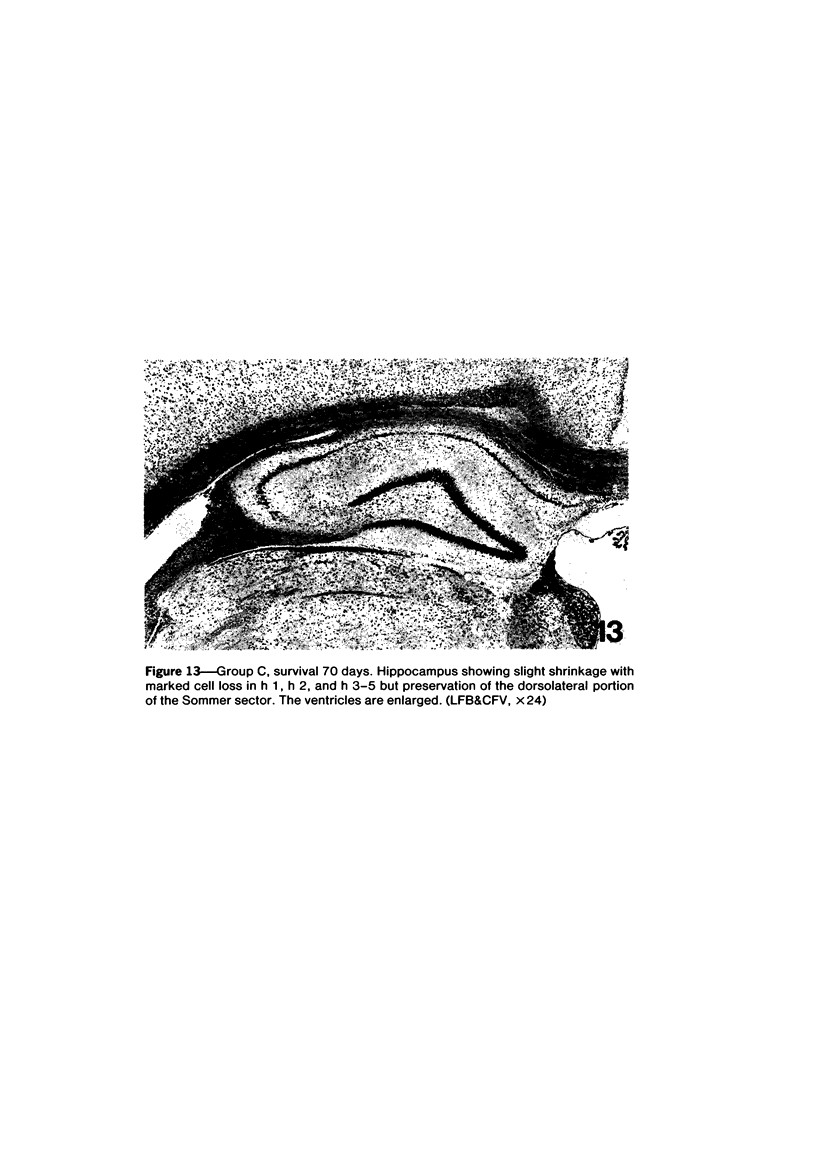
Trimethyltin, when given by gavage to rats, has an LD50 of 12.6 mg/kg. Signs of poisoning include tremors, hyperexcitability, aggressive behavior, weight loss, and convulsions. After single (10 mg/kg) or repeated weekly doses (a maximum of four) of 4 mg/kg, rats, up to a survival time of 70 days, were perfusion-fixed for light microscopy. Trimethyltin was assayed in brain and blood in rats after similar treatments. Trimethyltin is cumulative and persistent and binds with high affinity to hemoglobin. Trimethyltin, unlike triethyltin, does not produce white matter edema in rats but does cause bilateral and symmetrical neuronal alterations involving the hippocampus (largely sparing the Sommer sector), pyriform cortex, amygdaloid nucleus, and neocortex. The earliest alteration was loss or dispersal of Nissl substance, then clumping of nuclear chromatin, followed by shrinkage and fragmentation of the nucleus within shrunken eosinophilic cytoplasm. These changes were associated with approximately 1.4 microgram trimethyltin/g wet weight in brain tissue 1 day after the second dose of 4 mg/kg or 2 days after a single dose of 10 mg/kg. Signs of poisoning gradually disappeared, and 4 rats surviving 70 days appeared normal, although their brains had severe damage with cell loss in the hippocampi and each pyriform cortex. Treatment of rats with trimethyltin, therefore, provides a chronic preparation with consistent lesions in the hippocampus of use in other behavioral and neuroanatomic studies. (Am J Pathol 97:59--82, 1979).
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNES J. M., STONER H. B. The toxicology of tin compounds. Pharmacol Rev. 1959 Jun;11(2 Pt 1):211–231. [PubMed] [Google Scholar]
- BARNES J. M., STONER H. B. Toxic properties of some dialkyl and trialkyl tin salts. Br J Ind Med. 1958 Jan;15(1):15–22. doi: 10.1136/oem.15.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. W., Brierley J. B. The nature, distribution and earliest stages of anoxic-ischaemic nerve cell damage in the rat brain as defined by the optical microscope. Br J Exp Pathol. 1968 Apr;49(2):87–106. [PMC free article] [PubMed] [Google Scholar]
- Brown A. W., Levy D. E., Kublik M., Harrow J., Plum F., Brierley J. B. Selective chromatolysis of neurons in the gerbil brain: a possible consequence of "epileptic" activity produced by common carotid artery occlusion. Ann Neurol. 1979 Feb;5(2):127–138. doi: 10.1002/ana.410050206. [DOI] [PubMed] [Google Scholar]
- Brown A. W. Structural abnormalities in neurones. J Clin Pathol Suppl (R Coll Pathol) 1977;11:155–169. doi: 10.1136/jcp.s3-11.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAUJOLLE F., LESBRE M., MEYNIER D. Sur la toxicité du tétraméthylstannane et du tétraéthylstannane. C R Hebd Seances Acad Sci. 1954 Aug 18;239(7):556–558. [PubMed] [Google Scholar]
- COGGESHALL R. E., MACLEAN P. D. Hippocampal lesions following administration of 3-acetylpyridine. Proc Soc Exp Biol Med. 1958 Aug-Sep;98(4):687–689. doi: 10.3181/00379727-98-24152. [DOI] [PubMed] [Google Scholar]
- CREMER J. E. Biochemical studies on the toxicity of tetraethyl lead and other organo-lead compounds. Br J Ind Med. 1959 Jul;16:191–199. doi: 10.1136/oem.16.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREMER J. E., CALLAWAY S. Further studies on the toxicity of some tetra and trialkyl lead compounds. Br J Ind Med. 1961 Oct;18:277–282. doi: 10.1136/oem.18.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREMER J. E. The biochemistry of organotin compounds; the conversion of tetraethyltin into triethyltin in mammals. Biochem J. 1958 Apr;68(4):685–692. doi: 10.1042/bj0680685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortemps E., Amand G., Bomboir A., Lauwerys R., Laterre E. C. Trimethyltin poisoning. Report of two cases. Int Arch Occup Environ Health. 1978 Jan 27;41(1):1–6. doi: 10.1007/BF00377794. [DOI] [PubMed] [Google Scholar]
- Friede R. L. The histochemical architecture of the Ammon's horn as related to its selective vulnerability. Acta Neuropathol. 1966 Jan 14;6(1):1–13. doi: 10.1007/BF00691077. [DOI] [PubMed] [Google Scholar]
- GRAY S. J., STERLING K. Determination of circulating red cell volume by radioactive chromium. Science. 1950 Aug 11;112(2902):179–180. doi: 10.1126/science.112.2902.179. [DOI] [PubMed] [Google Scholar]
- Huggins R. A., Smith E. L., Deavers S. Cr 51-tagged red cell equilibration in dogs. Am J Physiol. 1966 Aug;211(2):283–287. doi: 10.1152/ajplegacy.1966.211.2.283. [DOI] [PubMed] [Google Scholar]
- Ito U., Spatz M., Walker J. T., Jr, Klatzo I. Experimental cerebral ischemia in mongolian gerbils. I. Light microscopic observations. Acta Neuropathol. 1975 Aug 27;32(3):209–223. doi: 10.1007/BF00696570. [DOI] [PubMed] [Google Scholar]
- LEVINE S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960 Jan;36:1–17. [PMC free article] [PubMed] [Google Scholar]
- LITTAU V. C., ALLFREY V. G., FRENSTER J. H., MIRSKY A. E. ACTIVE AND INACTIVE REGIONS OF NUCLEAR CHROMATIN AS REVEALED BY ELECTRON MICROSCOPE AUTORADIOGRAPHY. Proc Natl Acad Sci U S A. 1964 Jul;52:93–100. doi: 10.1073/pnas.52.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Brierley J. B., Plum F. Ischaemic brain damage in the gerbil in the absence of 'no-reflow'. J Neurol Neurosurg Psychiatry. 1975 Dec;38(12):1197–1205. doi: 10.1136/jnnp.38.12.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee-Russell S. M., Brown A. W., Brierley J. B. A combined light and electron microscope study of early anoxic-ischaemic cell change in rat brain. Brain Res. 1970 Jun 3;20(2):193–200. doi: 10.1016/0006-8993(70)90288-x. [DOI] [PubMed] [Google Scholar]
- O'Connor T. M., Wyttenbach C. R. Cell death in the embryonic chick spinal cord. J Cell Biol. 1974 Feb;60(2):448–459. doi: 10.1083/jcb.60.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay S. L., Billings-Gagliardi S., Chan-Palay V. Neuronal perikarya with dispersed, single ribosomes in the visual cortex of Macaca mulatta. J Cell Biol. 1974 Dec;63(3):1074–1089. doi: 10.1083/jcb.63.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilar G., Landmesser L. Ultrastructural differences during embryonic cell death in normal and peripherally deprived ciliary ganglia. J Cell Biol. 1976 Feb;68(2):339–356. doi: 10.1083/jcb.68.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. S., Aldridge W. N. The interaction of triethyltin with components of animal tissues. Biochem J. 1968 Feb;106(4):821–828. doi: 10.1042/bj1060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. S. Evidence for histidine in the triethyltin-binding site of rat haemoglobin. Biochem J. 1969 Jan;111(2):129–137. doi: 10.1042/bj1110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STONER H. B., BARNES J. M., DUFF J. I. Studies on the toxicity of alkyl tin compounds. Br J Pharmacol Chemother. 1955 Mar;10(1):16–25. doi: 10.1111/j.1476-5381.1955.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]