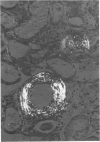
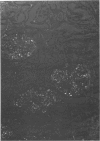
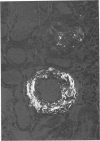
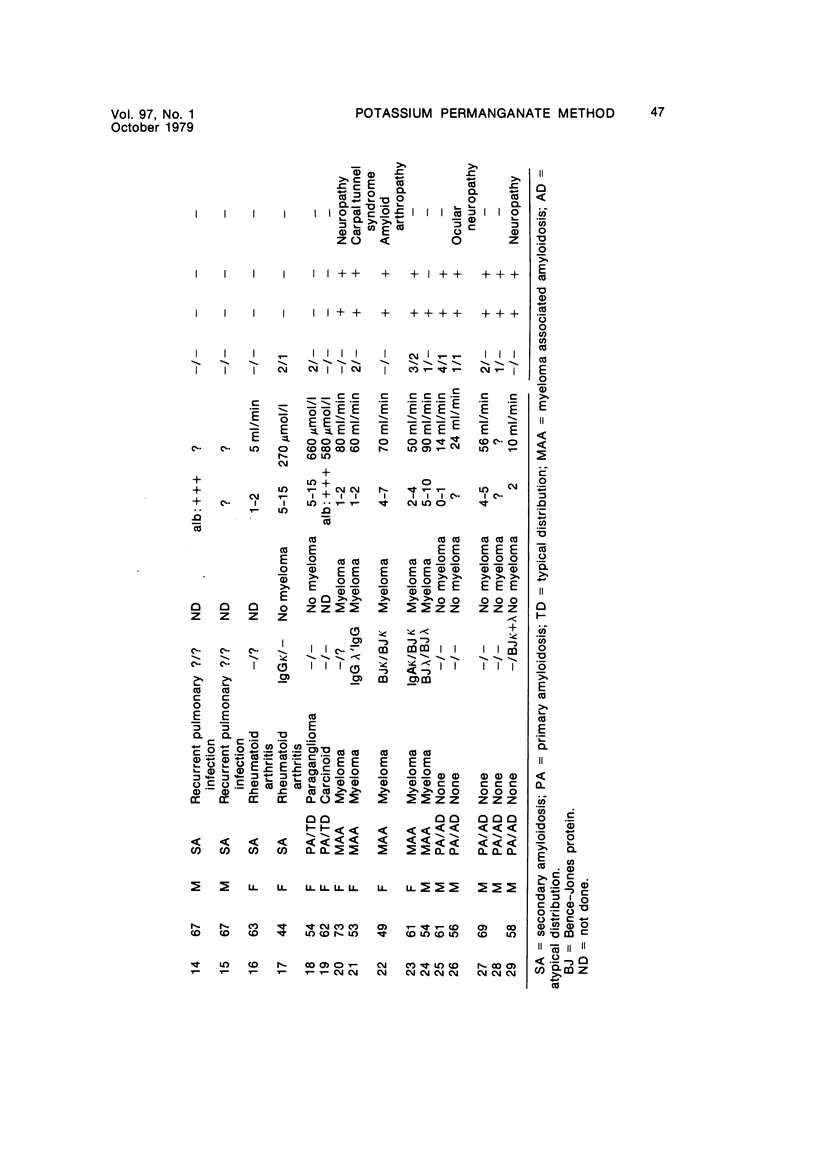
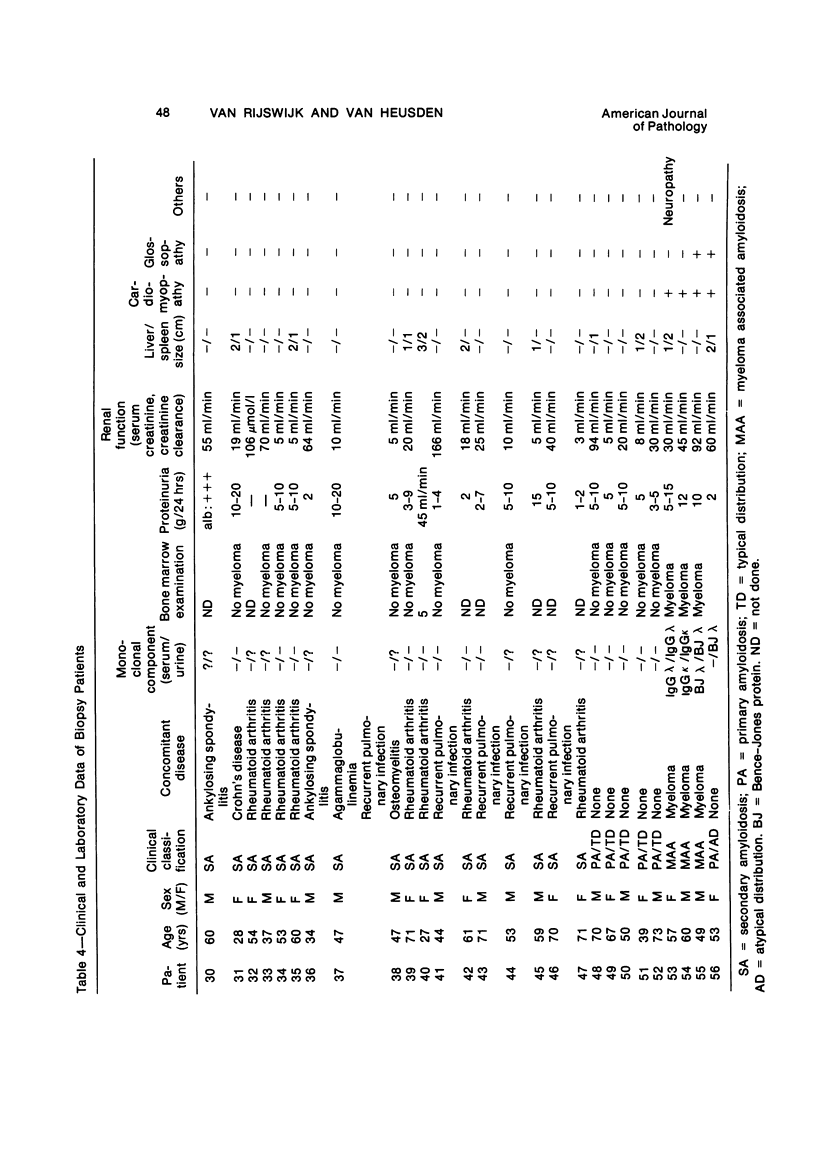
Abstract
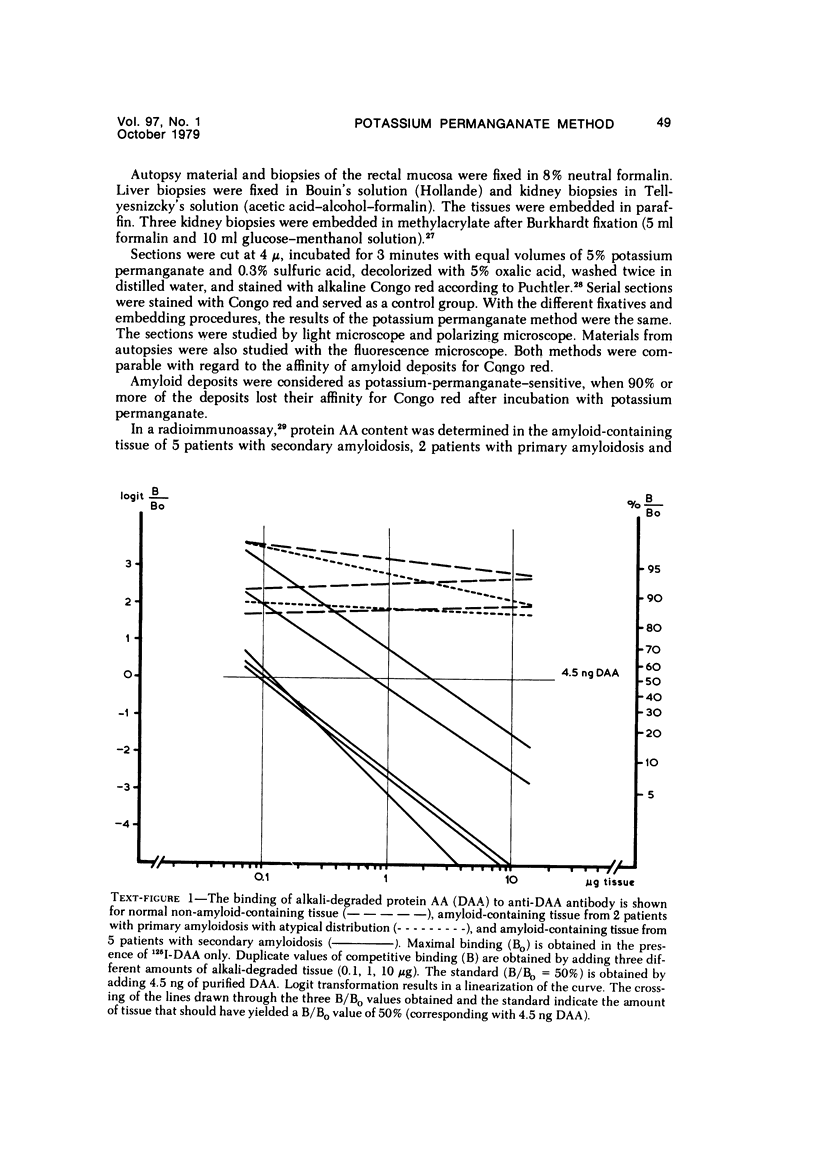
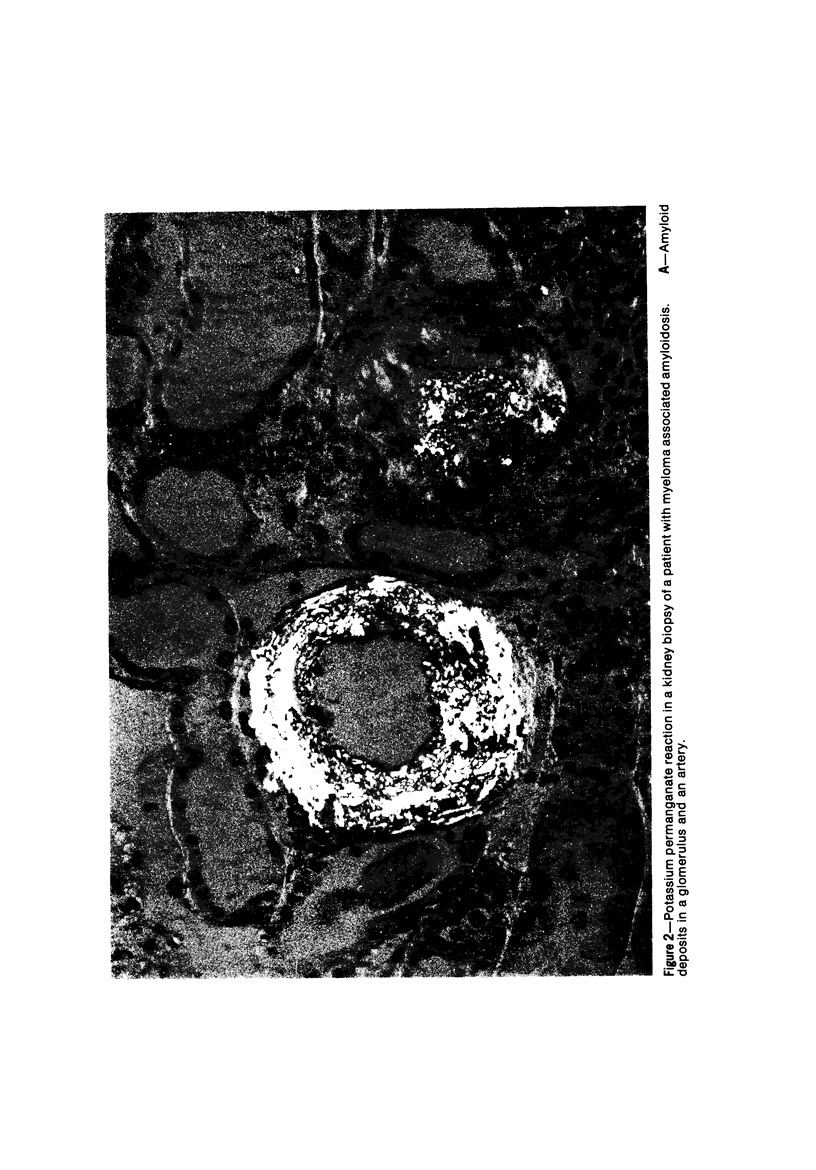
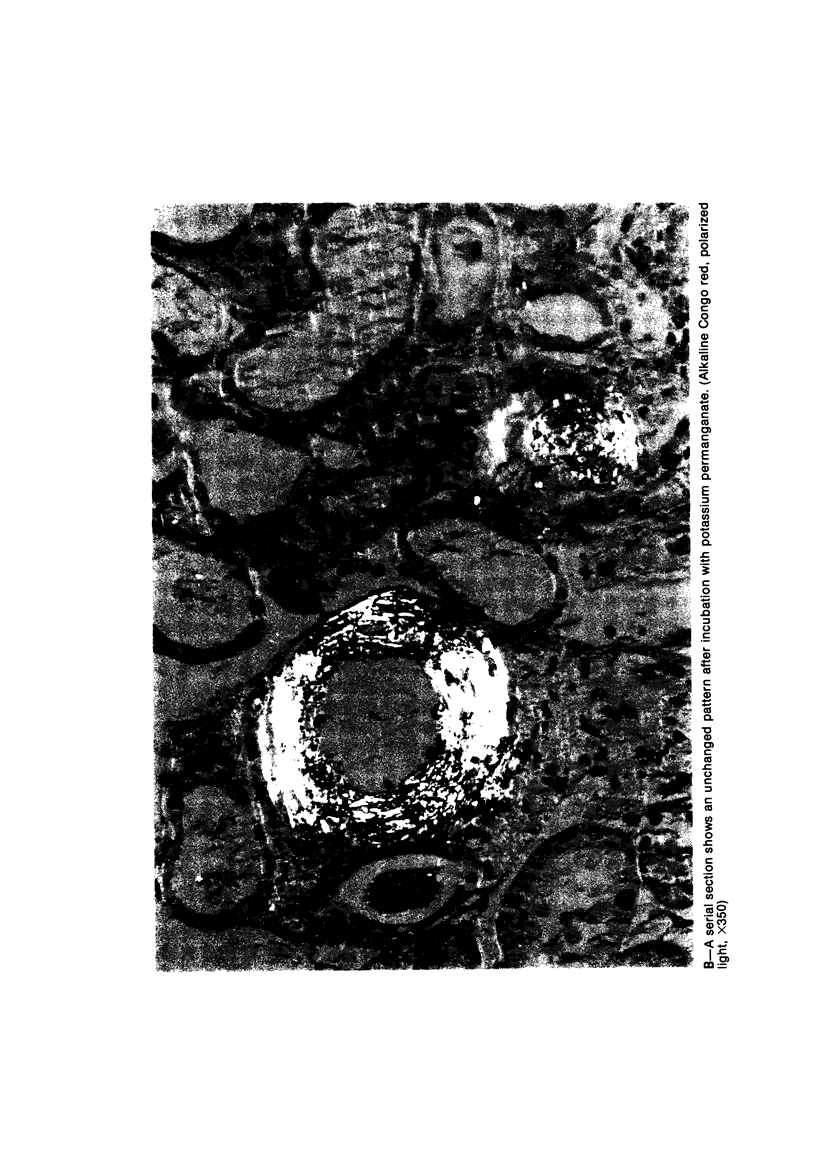
Alterations in affinity of amyloid for Congo red after incubation of tissue sections with potassium permanganate, as described by Wright el al, were studied. The affinity of amyloid for Congo red after incubation with potassium permanganate did not change in patients with myeloma-associated amyloidosis, familial amyloidotic polyneuropathy, medullary carcinoma of the thyroid, pancreatic island amyloid, and cerebral amyloidosis. Affinity for Congo red was lost after incubation with potassium permanganate in tissue sections from patients with secondary amyloidosis and amyloidosis complicating familial Mediterranean fever (consisting of amyloid AA). Patients with primary amyloidosis could be divided into two groups, one with potassium-permanganate--sensitive and one with potassium-permanganate--resistant amyloid deposits. These two groups correlated with the clinical classification in typical organ distribution (presenting with nephropathy) and atypical organ distribution (presenting with cardiomyopathy, nephropathy, and glossopathy) and the expected presence of amyloid AA or amyloid AL. Potassium permanganate sensitivity seems to be restricted to amyloid AA. The potassium permanganate method can be important in dividing the major forms of generalized amyloidosis in AA amyloid and non-AA amyloid. This can be used for differentiating early stages of the disease and cases otherwise difficult to classify. It is important to define patient groups properly, especially in evaluating the effect of therapeutic measures. (Am J Pathol 97:43--58, 1979).
Full text
PDF


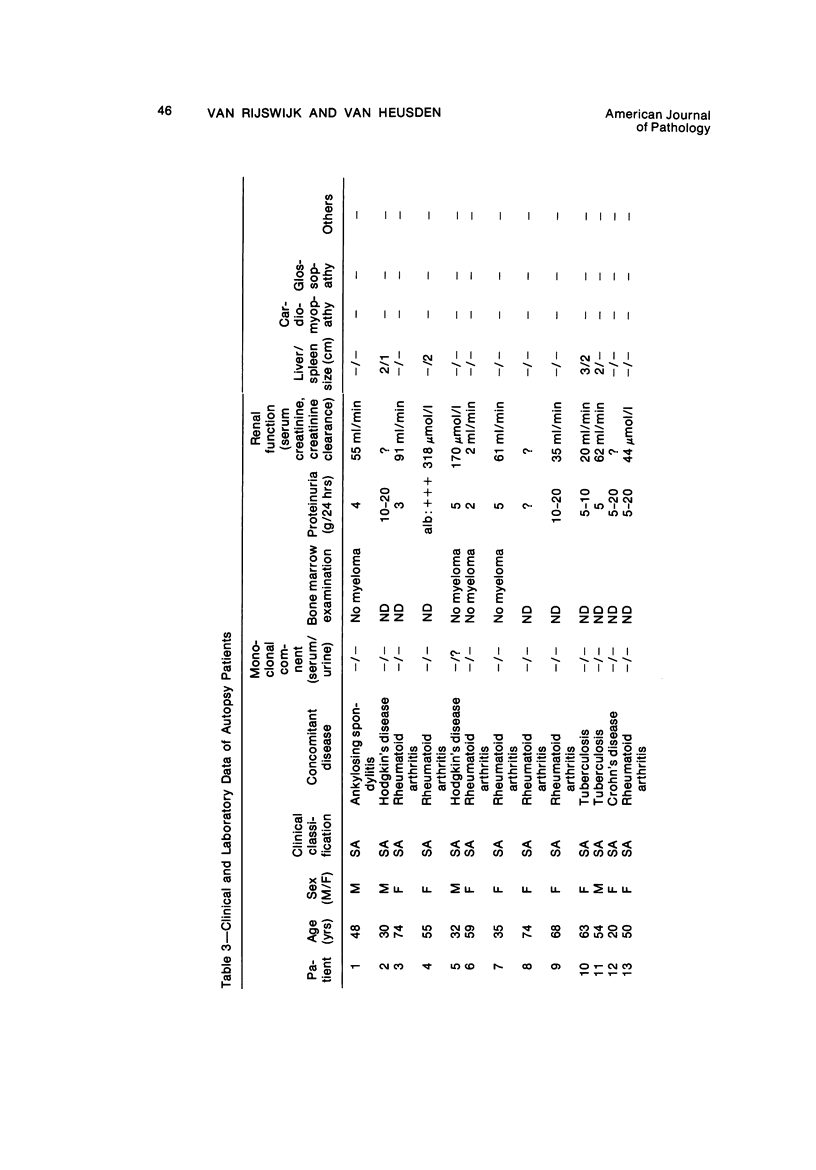







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRADE C. A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain. 1952 Sep;75(3):408–427. doi: 10.1093/brain/75.3.408. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Chemical similarity among amyloid substances associated with long standing inflammation. Lab Invest. 1972 Jun;26(6):615–625. [PubMed] [Google Scholar]
- Cooper J. H. Selective amyloid staining as a function of amyloid composition and structure. Histochemical analysis of the alkaline Congo red, standardized toluidine blue, and iodine methods. Lab Invest. 1974 Sep;31(3):232–238. [PubMed] [Google Scholar]
- Cornwell G. G., 3rd, Husby G., Westermark P., Natvig J. B., Michaelsen T. E., Skogen B. Identification and characterization of different amyloid fibril proteins in tissue sections. Scand J Immunol. 1977;6(11):1071–1080. doi: 10.1111/j.1365-3083.1977.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Costa P. P., Figueira A. S., Bravo F. R. Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4499–4503. doi: 10.1073/pnas.75.9.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERIKSEN T., GOTZSCHE H., HARBOE N., KIAER W., MELLEMGAARD K. Familial primary amyloidosis with severe amyloid heart disease. Am J Med. 1962 Sep;33:328–348. doi: 10.1016/0002-9343(62)90230-9. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Harbaugh J., Ohma J. I., Harada M., Cuatrecasas P. An amyloid protein: the amino-terminal variable fragment of an immunoglobulin light chain. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1287–1289. doi: 10.1016/0006-291x(70)90227-5. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Terry W., Harada M., Isersky C., Page D. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971 Jun 11;172(3988):1150–1151. doi: 10.1126/science.172.3988.1150. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Rosenthal C. J., Franklin E. C. Amyloid-related serum component (SAA)--studies in acute infections, medullary thyroid carcinoma, and postsurgery. Clin Immunol Immunopathol. 1976 Jul;6(1):83–93. doi: 10.1016/0090-1229(76)90063-5. [DOI] [PubMed] [Google Scholar]
- Husby G., Sletten K., Michaelsen T. E., Natvig J. B. Antigenic and chemical characterization of non-immunoglobulin amyloid proteins. Scand J Immunol. 1972;1(4):393–400. doi: 10.1111/j.1365-3083.1972.tb03305.x. [DOI] [PubMed] [Google Scholar]
- Isobe T., Osserman E. F. Patterns of amyloidosis and their association with plasma-cell dyscrasia, monoclonal immunoglobulins and Bence-Jones proteins. N Engl J Med. 1974 Feb 28;290(9):473–477. doi: 10.1056/NEJM197402282900902. [DOI] [PubMed] [Google Scholar]
- KING L. S. Atypical amyloid disease, with observations on a new silver stain for amyloid. Am J Pathol. 1948 Sep;24(5):1095–1115. [PMC free article] [PubMed] [Google Scholar]
- Kyle R. A., Bayrd E. D. Amyloidosis: review of 236 cases. Medicine (Baltimore) 1975 Jul;54(4):271–299. doi: 10.1097/00005792-197507000-00001. [DOI] [PubMed] [Google Scholar]
- Levin M., Franklin E. C., Frangione B., Pras M. The amino acid sequence of a major nonimmunoglobulin component of some amyloid fibrils. J Clin Invest. 1972 Oct;51(10):2773–2776. doi: 10.1172/JCI107098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUCKLE T. J., WELLSM Urticaria, deafness, and amyloidosis: a new heredo-familial syndrome. Q J Med. 1962 Apr;31:235–248. [PubMed] [Google Scholar]
- Mahloudji M., Teasdall R. D., Adamkiewicz J. J., Hartmann W. H., Lambird P. A., McKusick V. A. The genetic amyloidoses with particular reference to hereditary neuropathic amyloidosis, type II (Indiana or Rukavina type). Medicine (Baltimore) 1969 Jan;48(1):1–37. [PubMed] [Google Scholar]
- Ravid M., Keizman I. K., Sohar E. Effect of a single dose of dimethyl sulphoxide on renal amyloidosis. Lancet. 1977 Apr 2;1(8014):730–731. doi: 10.1016/s0140-6736(77)92171-7. [DOI] [PubMed] [Google Scholar]
- Ravid M., Robson M., Kedar I. Prolonged colchicine treatment in four patients with amyloidosis. Ann Intern Med. 1977 Nov;87(5):568–570. doi: 10.7326/0003-4819-87-5-568. [DOI] [PubMed] [Google Scholar]
- Reimann H. A., Koucky R. F., Eklund C. M. Primary Amyloidosis Limited to Tissue of Mesodermal Origin. Am J Pathol. 1935 Nov;11(6):977–988.3. [PMC free article] [PubMed] [Google Scholar]
- Romhányi G. Differences in ultrastructural organization of amyloid as revealed by sensitivity or resistance to induced proteolysis. Virchows Arch A Pathol Pathol Anat. 1972;357(1):29–52. doi: 10.1007/BF00548215. [DOI] [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C. Variation with age and disease of an amyloid A protein-related serum component. J Clin Invest. 1975 Apr;55(4):746–753. doi: 10.1172/JCI107985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe J. D., Ignaczak T. F., Pollock P. S., Glenner G. G. Amyloid fibril protein AA: purification and properties of the antigenically related serum component as determined by solid phase radioimmunoassay. J Immunol. 1976 Apr;116(4):1151–1156. [PubMed] [Google Scholar]
- Sletten K., Westermark P., Natvig J. B. Characterization of amyloid fibril proteins from medullary carcinoma of the thyroid. J Exp Med. 1976 Apr 1;143(4):993–998. doi: 10.1084/jem.143.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Natvig J. B., Anders R. F., Sletten K., Husby G. Coexistence of protein AA and immunoglobulin light-chain fragments in amyloid fibrils. Scand J Immunol. 1976;5(1-2):31–36. doi: 10.1111/j.1365-3083.1976.tb02989.x. [DOI] [PubMed] [Google Scholar]
- Westermark P., Natvig J. B., Johansson B. Characterization of an amyloid fibril protein from senile cardiac amyloid. J Exp Med. 1977 Aug 1;146(2):631–636. doi: 10.1084/jem.146.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman J. E., Delleman J. W., Ansink B. J. Ein hereditäres Syndrom, bestehend aus peripherer Polyneuopatie, Hauveränderungen und gittriger Dystrophie der Hornhaut. Klin Monbl Augenheilkd. 1971 Nov;159(5):618–623. [PubMed] [Google Scholar]
- Wright J. R., Calkins E., Humphrey R. L. Potassium permanganate reaction in amyloidosis. A histologic method to assist in differentiating forms of this disease. Lab Invest. 1977 Mar;36(3):274–281. [PubMed] [Google Scholar]
- van Rijswijk M. H., Donker A. J., Ruinen L. Dimethylsulphoxide in amyloidosis. Lancet. 1979 Jan 27;1(8109):207–208. doi: 10.1016/s0140-6736(79)90598-1. [DOI] [PubMed] [Google Scholar]