Abstract
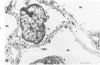
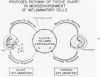
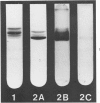
Human polymorphonuclear neutrophilic leukocytes (PMNs) contain large amounts of neutral proteases that can degrade elastin, collagen, proteoglycan, and basement membrane. The instillation of one of the purified enzymes (elastase) into dog lungs in vivo causes degradation of elastic fibers and other alveolar septal components and results in anatomic changes similar to those of human pulmonary emphysema. Cigarette smoking is a major risk factor associated with pulmonary emphysema in man. One mechanism for this association may be interference with the regulation of PMN elastase activity by alveolar antiproteases. This possibility is supported by the observation that the oxidizing activity of tobacco smoke inactivates alpha 1-proteinase inhibitor in vitro. Macrophages also secrete an elastolytic protease, albeit at low levels. The short-term exposure of cultured mouse macrophages to cigarette smoke augments the rate of elastase secretion by these cells. Mouse macrophage elastase is not inhibited by alpha 1-proteinase inhibitor or alpha 2-macroglobulin. This unusual property of macrophage elastase may facilitate its attack upon elastin over prolonged intervals despite very low levels of macrophage elastase production. A unified hypothesis of lung injury in pulmonary emphysema is presented, involving both PMN and macrophage elastases and the actions of cigarette smoke. (Am J Pathol 97:111--136, 1979).
Full text
PDF












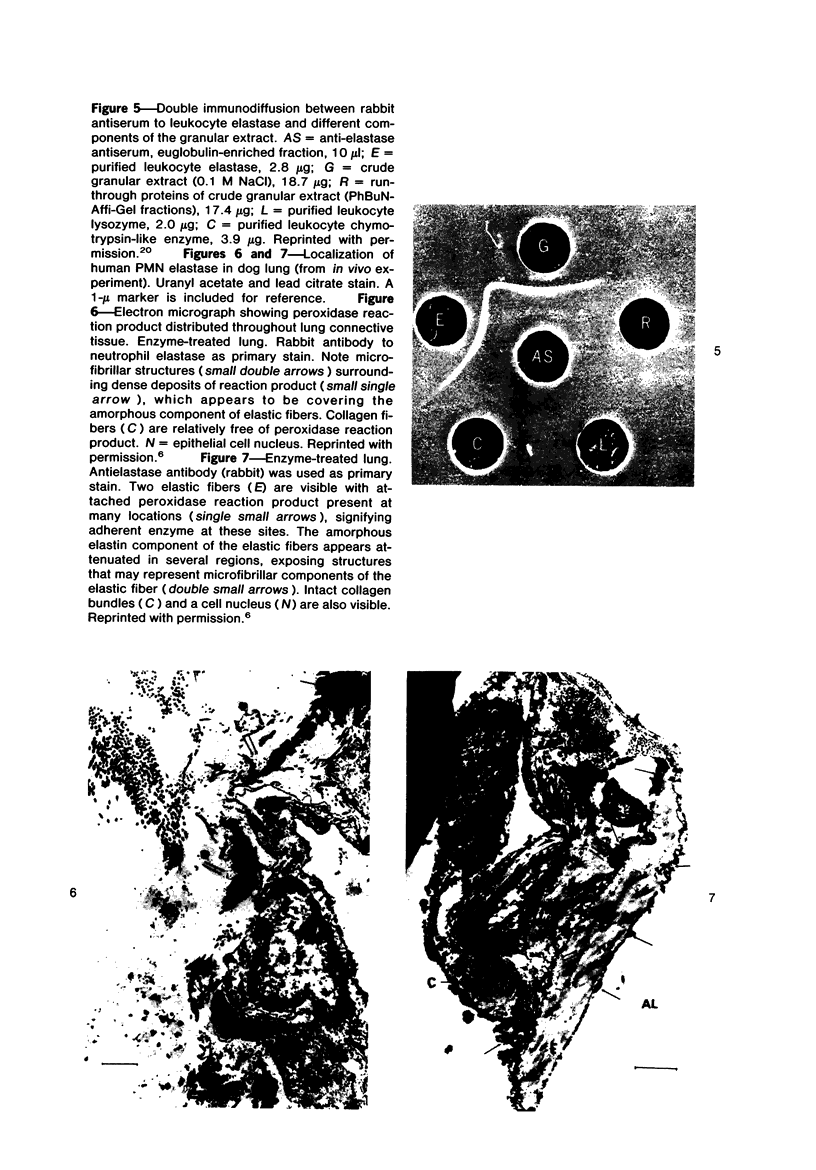

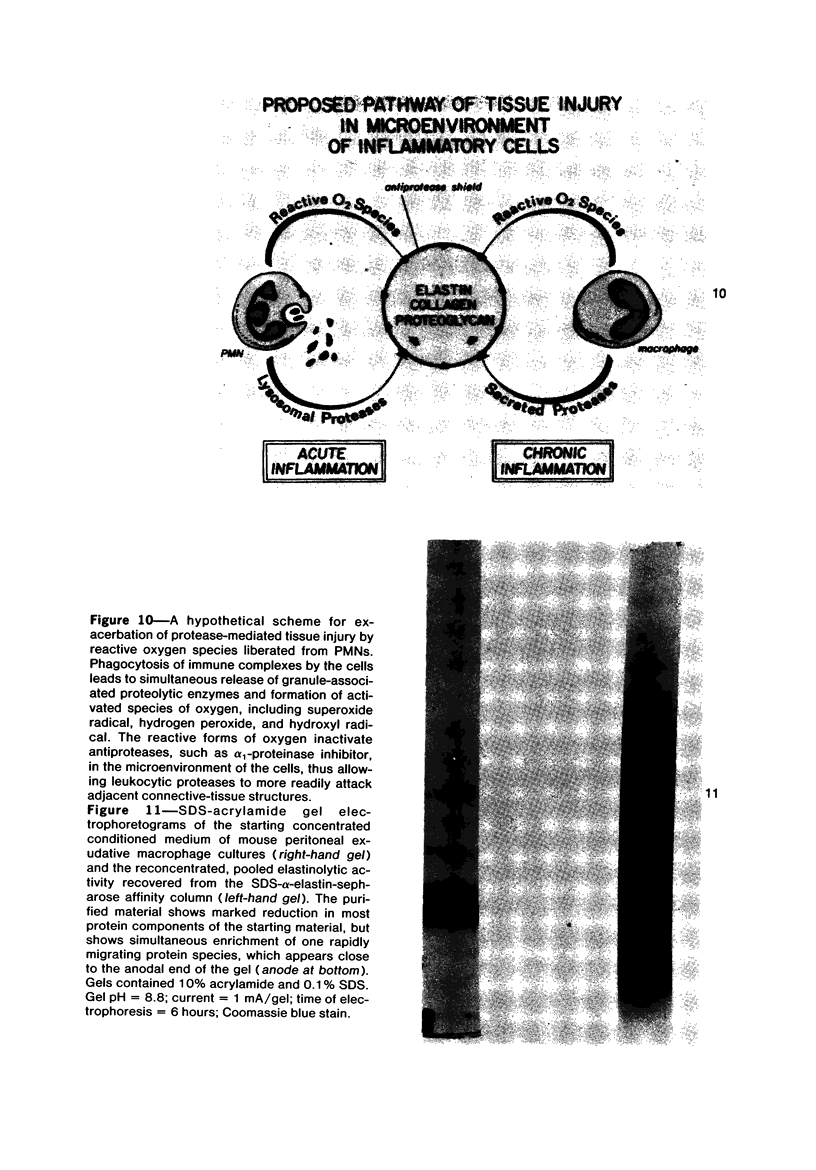

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach O., Hammond E. C., Garfinkel L., Benante C. Relation of smoking and age to emphysema. Whole-lung section study. N Engl J Med. 1972 Apr 20;286(16):853–857. doi: 10.1056/NEJM197204202861601. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Bretz U., Dewald B., Feigenson M. E. The polymorphonuclear leukocyte. Agents Actions. 1978 Jan;8(1-2):3–10. doi: 10.1007/BF01972395. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. The possible role of neutrophil proteinases in damage to articular cartilage. Agents Actions. 1978 Jan;8(1-2):11–18. doi: 10.1007/BF01972396. [DOI] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978 Sep;118(3):617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- Cohen A. B. The effects in vivo and in vitro of oxidative damage to purified alpha1-antitrypsin and to the enzyme-inhibiting activity of plasma. Am Rev Respir Dis. 1979 Jun;119(6):953–960. doi: 10.1164/arrd.1979.119.6.953. [DOI] [PubMed] [Google Scholar]
- Feinstein G., Janoff A. A rapid method of purification of human granulocyte cationic neutral proteases: purification and further characterization of human granulocyte elastase. Biochim Biophys Acta. 1975 Oct 22;403(2):493–505. doi: 10.1016/0005-2744(75)90077-7. [DOI] [PubMed] [Google Scholar]
- Gordon S., Newman W., Bloom B. Macrophage proteases and rheumatic diseases: regulation of plasminogen activator by thymus-derived lymphocytes. Agents Actions. 1978 Jan;8(1-2):19–26. doi: 10.1007/BF01972397. [DOI] [PubMed] [Google Scholar]
- Gordon S., Unkeless J. C., Cohn Z. A. Induction of macrophage plasminogen activator by endotoxin stimulation and phagocytosis: evidence for a two-stage process. J Exp Med. 1974 Oct 1;140(4):995–1010. doi: 10.1084/jem.140.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. Interaction of cells with immune complexes: adherence, release of constituents, and tissue injury. J Exp Med. 1971 Sep 1;134(3 Pt 2):114s–135s. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971 Dec;107(6):1547–1557. [PubMed] [Google Scholar]
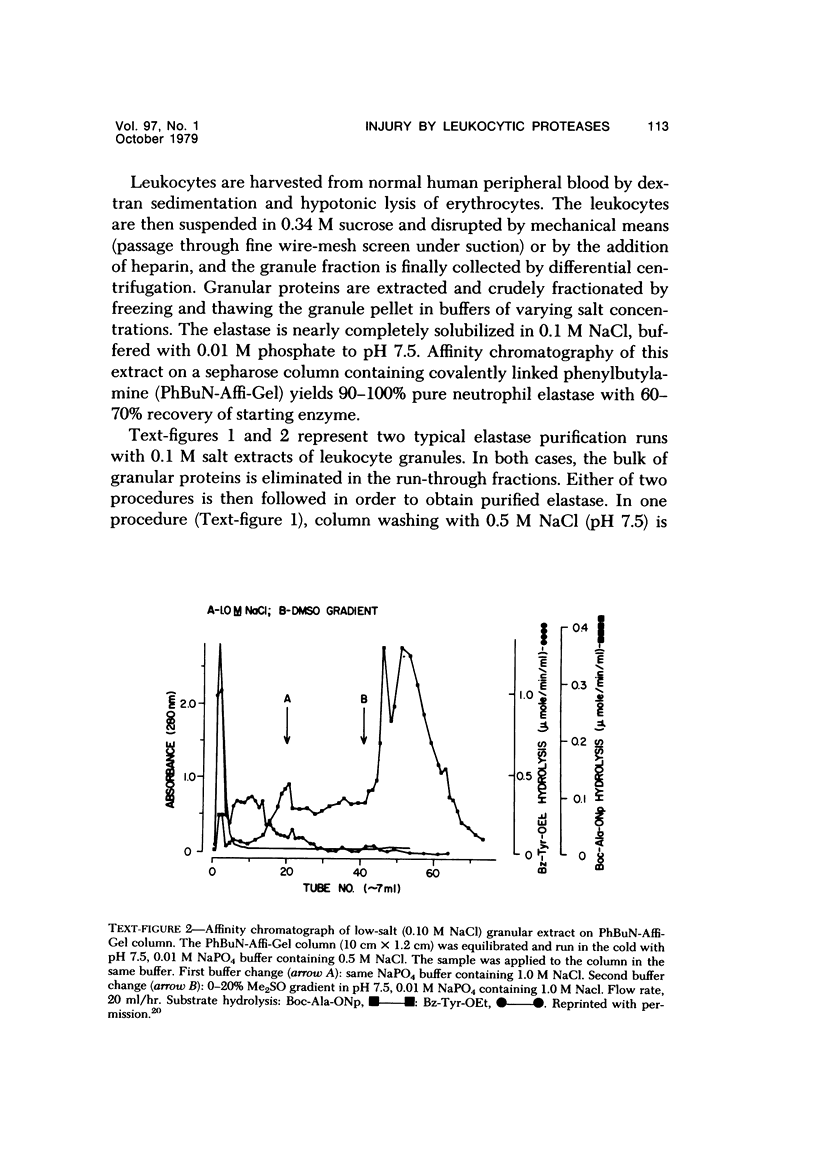
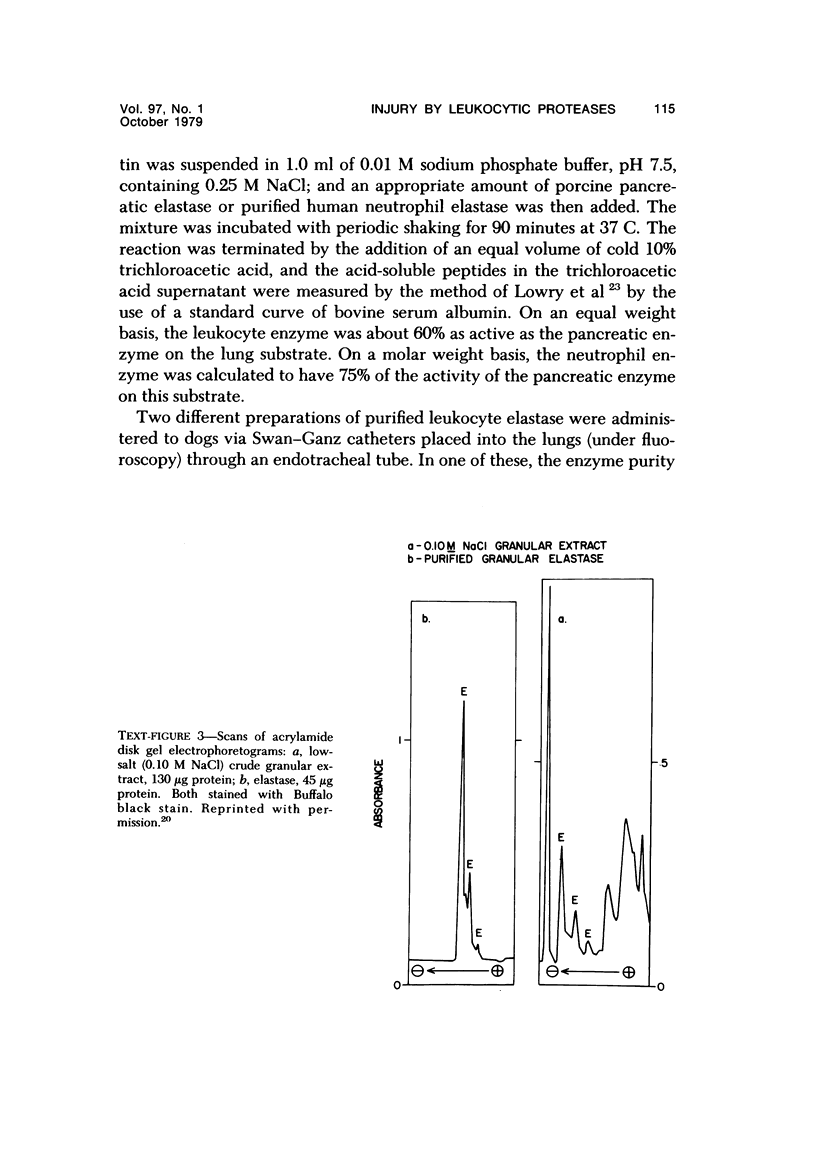
- Janoff A., Feinstein G., Malemud C. J., Elias J. M. Degradation of cartilage proteoglycan by human leukocyte granule neutral proteases--a model of joint injury. I. Penetration of enzyme into rabbit articular cartilage and release of 35SO4-labeled material from the tissue. J Clin Invest. 1976 Mar;57(3):615–624. doi: 10.1172/JCI108317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A. Mediators of tissue damage in leukocyte lysosomes. X. Further studies on human granulocyte elastase. Lab Invest. 1970 Mar;22(3):228–236. [PubMed] [Google Scholar]
- Janoff A. Purification of human granulocyte elastase by affinity chromatography. Lab Invest. 1973 Oct;29(4):458–464. [PubMed] [Google Scholar]
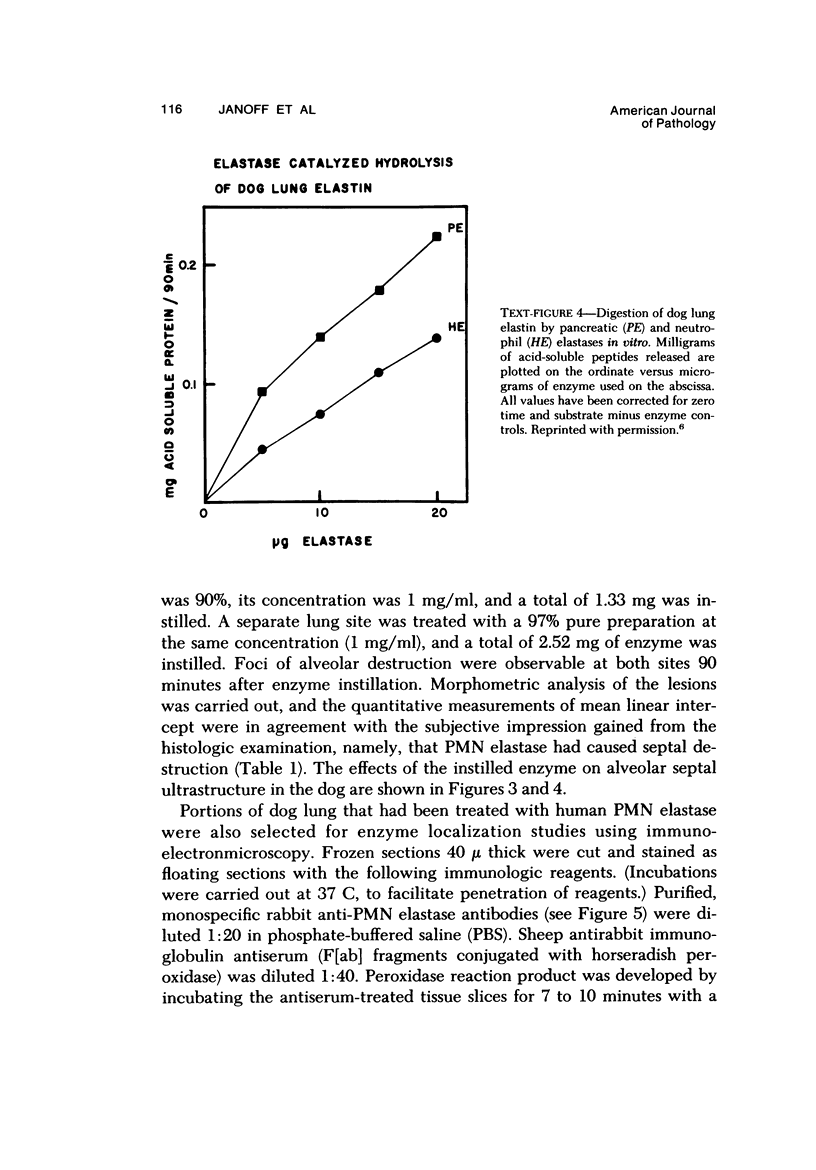
- Janoff A., Sandhaus R. A., Hospelhorn V. D., Rosenberg R. Digestion of lung proteins by human leukocyte granules in vitro. Proc Soc Exp Biol Med. 1972 Jun;140(2):516–519. doi: 10.3181/00379727-140-36493. [DOI] [PubMed] [Google Scholar]
- Janoff A., Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968 Nov 1;128(5):1137–1155. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Sloan B., Weinbaum G., Damiano V., Sandhaus R. A., Elias J., Kimbel P. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis. 1977 Mar;115(3):461–478. doi: 10.1164/arrd.1977.115.3.461. [DOI] [PubMed] [Google Scholar]
- Johnson D., Travis J. Structural evidence for methionine at the reactive site of human alpha-1-proteinase inhibitor. J Biol Chem. 1978 Oct 25;253(20):7142–7144. [PubMed] [Google Scholar]
- Keiser H., Greenwald R. A., Feinstein G., Janoff A. Degradation of cartilage proteoglycan by human leukocyte granule neutral proteases--a model of joint injury. II. Degradation of isolated bovine nasal cartilage proteoglycan. J Clin Invest. 1976 Mar;57(3):625–632. doi: 10.1172/JCI108318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimetzek V., Sorg C. Lymphokine-induced secretion of plasminogen activator by murine macrophages. Eur J Immunol. 1977 Mar;7(3):185–187. doi: 10.1002/eji.1830070314. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarus G. S., Daniels J. R., Brown R. S., Bladen H. A., Fullmer H. M. Degradation of collagen by a human granulocyte collagenolytic system. J Clin Invest. 1968 Dec;47(12):2622–2629. doi: 10.1172/JCI105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Gordon S., Cohn Z. Trypanosoma cruzi: the immunological induction of macrophage plasminogen activator requires thymus-derived lymphocytes. J Exp Med. 1977 Jul 1;146(1):172–183. doi: 10.1084/jem.146.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of granulocyte elastases. Eur J Biochem. 1974 Mar 1;42(2):519–527. doi: 10.1111/j.1432-1033.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., White R. R., Senior R. M., Levine E. A. Elastase release from human alveolar macrophages: comparison between smokers and nonsmokers. Science. 1977 Oct 21;198(4314):313–314. doi: 10.1126/science.910131. [DOI] [PubMed] [Google Scholar]
- Satoh S., Kurecki T., Kress L. F., Laskowski M., Sr The dual nature of the reaction between porcine elastase and human plasma alpha1 proteinase inhibitor. Biochem Biophys Res Commun. 1979 Jan 15;86(1):130–137. doi: 10.1016/0006-291x(79)90391-7. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Havemann K. Isolation of elastase-like and chymotrypsin-like neutral proteases from human granulocytes. Hoppe Seylers Z Physiol Chem. 1974 Sep;355(9):1077–1082. doi: 10.1515/bchm2.1974.355.2.1077. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Burnstein Y., Gertler A. Effect of oxidation of methionine residues in chicken ovoinhibitor on its inhibitory activities against trypsin, chymotrypsin, and elastase. Biochemistry. 1977 Mar 8;16(5):992–997. doi: 10.1021/bi00624a029. [DOI] [PubMed] [Google Scholar]
- Stedman R. L. The chemical composition of tobacco and tobacco smoke. Chem Rev. 1968 Apr;68(2):153–207. doi: 10.1021/cr60252a002. [DOI] [PubMed] [Google Scholar]
- TONGE B. L. Effect of tobacco smoke condensate on the aerial oxidation of cysteine. Nature. 1962 Apr 21;194:284–285. doi: 10.1038/194284a0. [DOI] [PubMed] [Google Scholar]
- Taylor J. C., Crawford I. P. Purification and preliminary characterization of human leukocyte elastasel. Arch Biochem Biophys. 1975 Jul;169(1):91–101. doi: 10.1016/0003-9861(75)90320-3. [DOI] [PubMed] [Google Scholar]
- Tuttle W. C., Westerberg S. C. Alpha-1 globulin trypsin inhibitor in canine surfactant protein. Proc Soc Exp Biol Med. 1974 May;146(1):232–235. doi: 10.3181/00379727-146-38076. [DOI] [PubMed] [Google Scholar]
- Twumasi D. Y., Liener I. E. Proteases from purulent sputum. Purification and properties of the elastase and chymotrypsin-like enzymes. J Biol Chem. 1977 Mar 25;252(6):1917–1926. [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Reich E. Macrophage plasminogen activator: induction by products of activated lymphoid cells. J Exp Med. 1977 Feb 1;145(2):429–437. doi: 10.1084/jem.145.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by lymphokine-activated macrophages. Science. 1975 Jan 24;187(4173):261–263. doi: 10.1126/science.163038. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
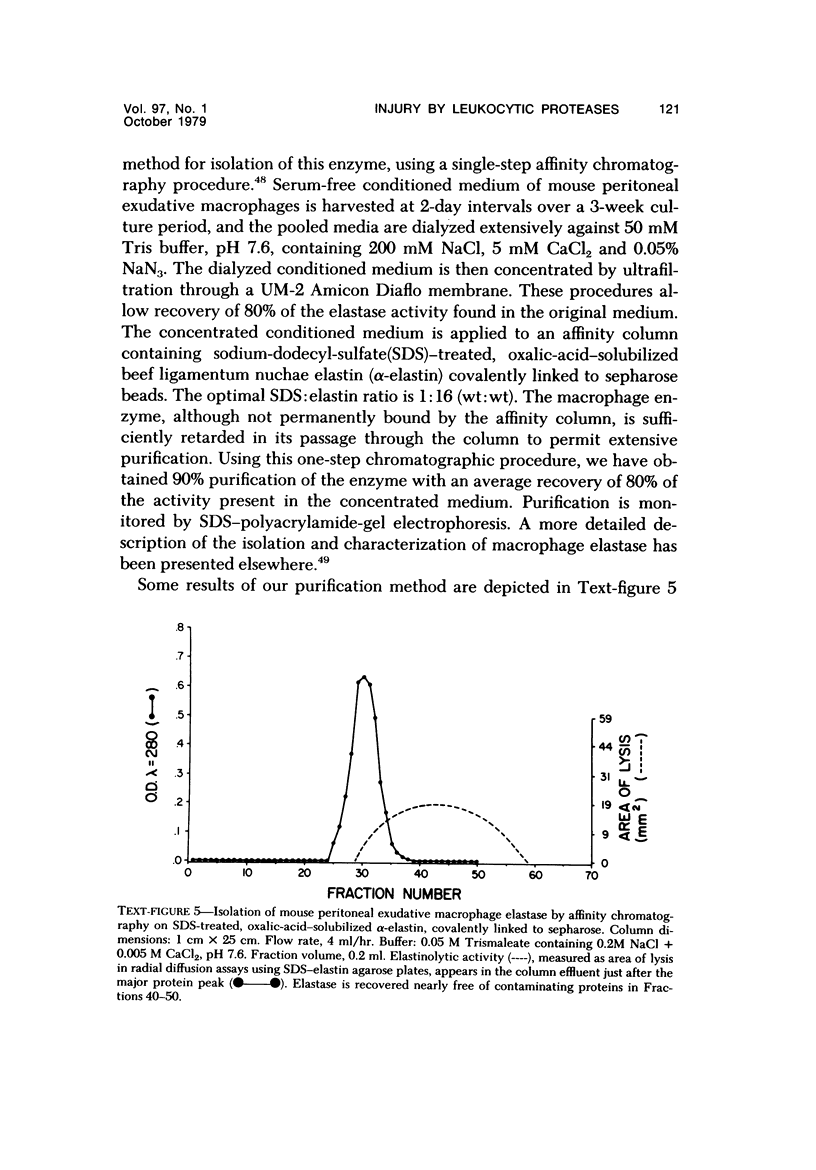
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- White R., Lin H. S., Kuhn C., 3rd Elastase secretion by peritoneal exudative and alveolar macrophages. J Exp Med. 1977 Sep 1;146(3):802–808. doi: 10.1084/jem.146.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. G., Malawista S. E. The mobilization and extracellular release of granular enzymes from human leukocytes during phagocytosis. J Cell Biol. 1972 Jun;53(3):788–797. doi: 10.1083/jcb.53.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]