Abstract
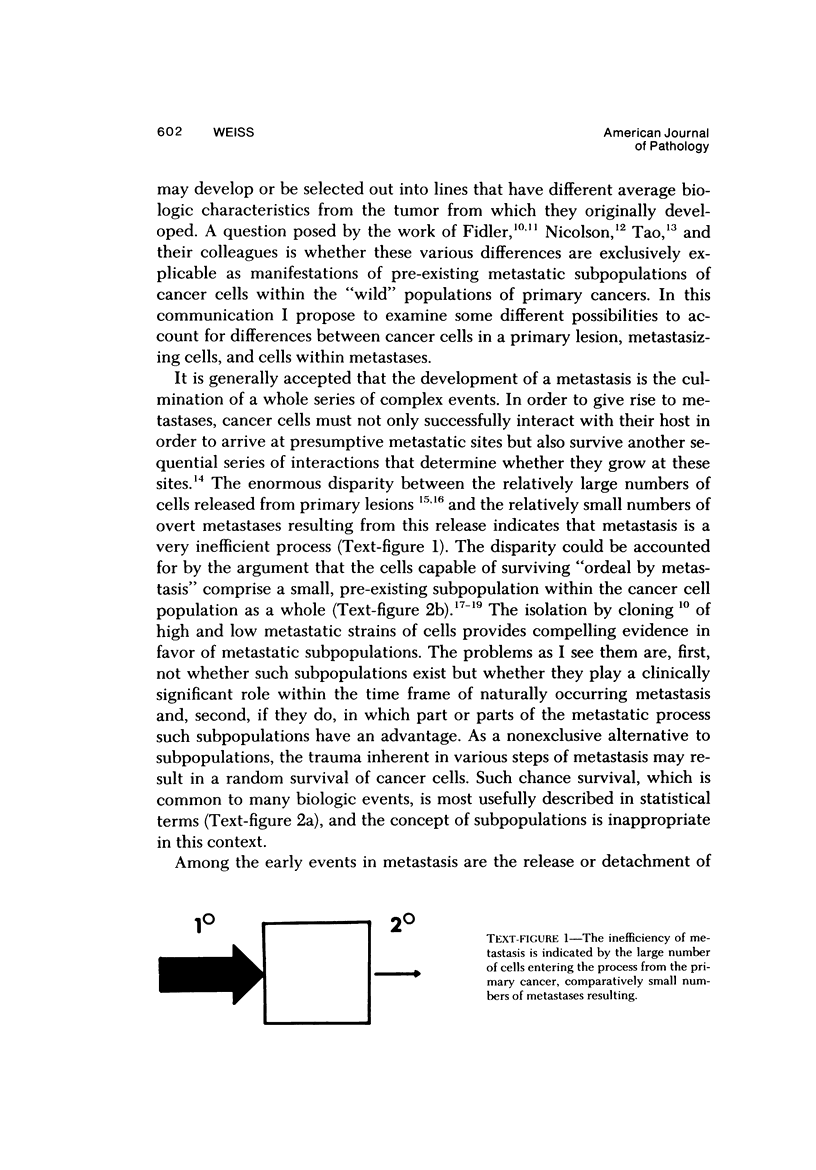
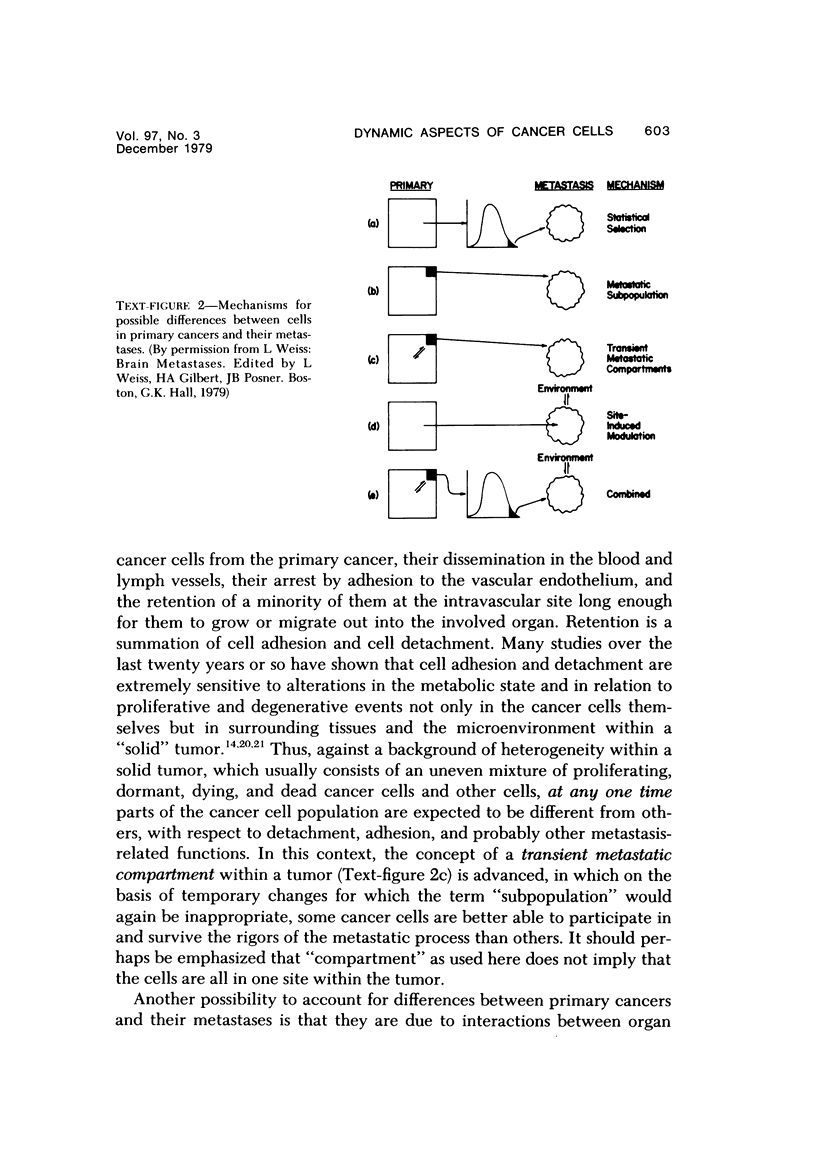
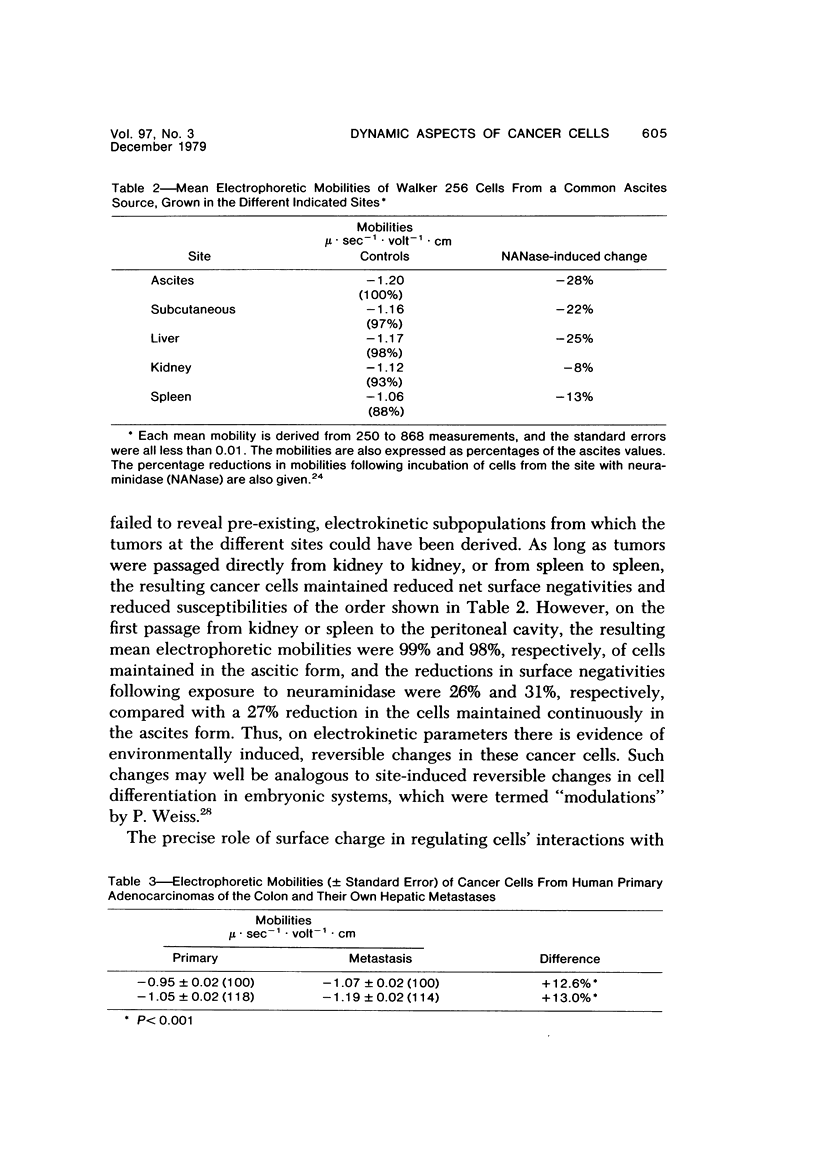
Consideration of the entire metastatic process reveals it to be very inefficient in terms of cancer cells. Of the millions of cells released from primary cancers, relatively few metastases result. This disparity implies that in some way the process is selective. Some evidence will be reviewed that indicates that cancer cells in metastases are in some way different from those in the primary cancer from which they arose. Primary cancers and their metastases, then, should possibly be regarded as distinct entities when one is considering therapy or seeking an understanding of the fundamental aspects of metastasis. In this presentation some nonexclusive mechanisms will be discussed that could be responsible for differences between primary and secondary cancers. These include: 1) Random (statistical) selection of metastasis-forming cells; 2) The existence of genotypic metastatic subpopulations; 3) The existence of transient metastatic "compartments" within primary cancer; 4) Site-induced changes (modulation) occurring in the metastasizing cells after they arrive in the target organ; 5) A combination of the above.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellamy D., Hinsull S. M. Influence of lodgement site on the proliferation of metastases of Walker 256 carcinoma in the rat. Br J Cancer. 1978 Jan;37(1):81–85. doi: 10.1038/bjc.1978.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson K. W., Nicolson G. L. Selection and biologic properties of malignant variants of a murine lymphosarcoma. J Natl Cancer Inst. 1978 Dec;61(6):1499–1503. [PubMed] [Google Scholar]
- Butler T. P., Gullino P. M. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 1975 Mar;35(3):512–516. [PubMed] [Google Scholar]
- COOK G. M., SEAMAN G. V., WEISS L. PHYSICOCHEMICAL DIFFERENCES BETWEEN ASCITIC AND SOLID FORMS OF SARCOMA 37 CELLS. Cancer Res. 1963 Dec;23:1813–1818. [PubMed] [Google Scholar]
- Donelli M. G., Colombo T., Broggini M., Garattini S. Differential distribution of antitumor agents in primary and secondary tumors. Cancer Treat Rep. 1977 Oct;61(7):1319–1324. [PubMed] [Google Scholar]
- EISENBERG S., BEN-OR S., DOLJANSKI F. Electro-kinetic properties of cells in growth processes. I. The electrophoretic behavior of liver cells during regeneration and post-natal growth. Exp Cell Res. 1962 Mar;26:451–461. doi: 10.1016/0014-4827(62)90151-9. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Kripke M. L. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977 Aug 26;197(4306):893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- Fugmann R. A., Anderson J. C., Stolfi R. L., Martin D. S. Comparison of adjuvant chemotherapeutic activity against primary and metastatic spontaneous murine tumors. Cancer Res. 1977 Feb;37(2):496–500. [PubMed] [Google Scholar]
- LASNITZKI I. The behaviour of ascites tumour cells in vitro and in vivo. Br J Cancer. 1953 Jun;7(2):238–249. doi: 10.1038/bjc.1953.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane M. Clinical problems of resistance to cancer chemotherapeutic agents. Fed Proc. 1979 Jan;38(1):103–107. [PubMed] [Google Scholar]
- Liotta L. A., Kleinerman J., Saidel G. M. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 1974 May;34(5):997–1004. [PubMed] [Google Scholar]
- RABOTTI G. Ploidy of primary and metastatic human tumors. Nature. 1959 May 2;183(4670):1276–1277. doi: 10.1038/1831276b0. [DOI] [PubMed] [Google Scholar]
- Sandberg A. A., Yamada K. Chromosomes and causation of human cancer and leukemia. I. Karyotypic diversity in a single cancer. Cancer. 1966 Dec;19(12):1869–1878. doi: 10.1002/1097-0142(196612)19:12<1869::aid-cncr2820191214>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Slack N. H., Bross I. D. The influence of site of metastasis on tumour growth and response to chemotherapy. Br J Cancer. 1975 Jul;32(1):78–86. doi: 10.1038/bjc.1975.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T. W., Burger M. M. Non-metastasising variants selected from metastasising melanoma cells. Nature. 1977 Dec 1;270(5636):437–438. doi: 10.1038/270437a0. [DOI] [PubMed] [Google Scholar]
- Tropé C. Different sensitivity to cytostatic drugs of primary tumor and metastasis of the Lewis carcinoma. Neoplasma. 1975;22(2):171–180. [PubMed] [Google Scholar]
- Weiss L., Hakala M. T. The anionic nature of sarcoma 180 cell surfaces, and sensitivity to 4,4'-diacetyldiphenylurea-bis(guanylhydrazone). Cancer Res. 1971 Oct;31(10):1369–1372. [PubMed] [Google Scholar]
- Weiss L., Ratcliffe T. M. Effects of chemotherapeutic and other agents on cellular electrophoretic mobility. J Natl Cancer Inst. 1968 Oct;41(4):957–966. [PubMed] [Google Scholar]
- Weiss L. The cell periphery and metastasis. Proc Can Cancer Conf. 1967;7:292–315. [PubMed] [Google Scholar]
- Yamada K., Takagi N., Sandberg A. A. Chromosomes and causation of human cancer and leukemia. II. Karyotypes of human solid tumors. Cancer. 1966 Dec;19(12):1879–1890. doi: 10.1002/1097-0142(196612)19:12<1879::aid-cncr2820191215>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- ZEIDMAN I. THE FATE OF CIRCULATING TUMOR CELLS. Acta Cytol. 1965 Mar-Apr;9:136–140. [PubMed] [Google Scholar]


