Abstract
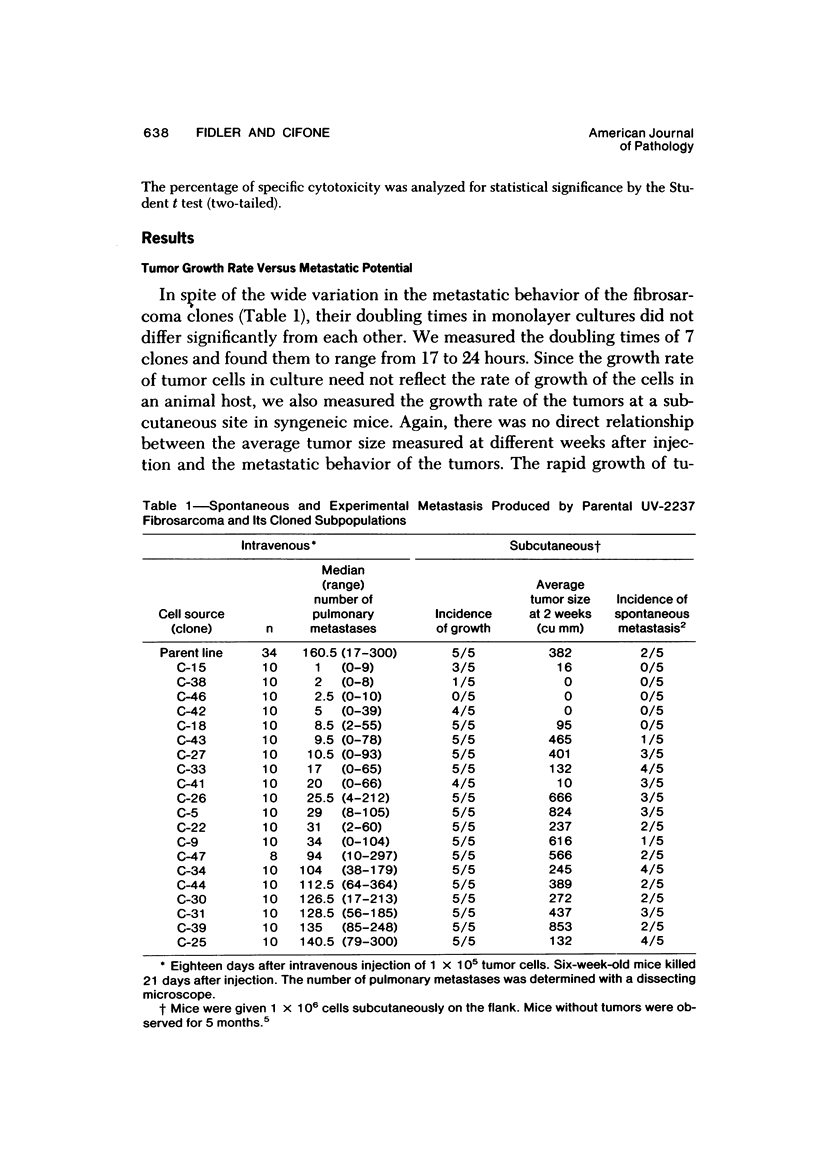
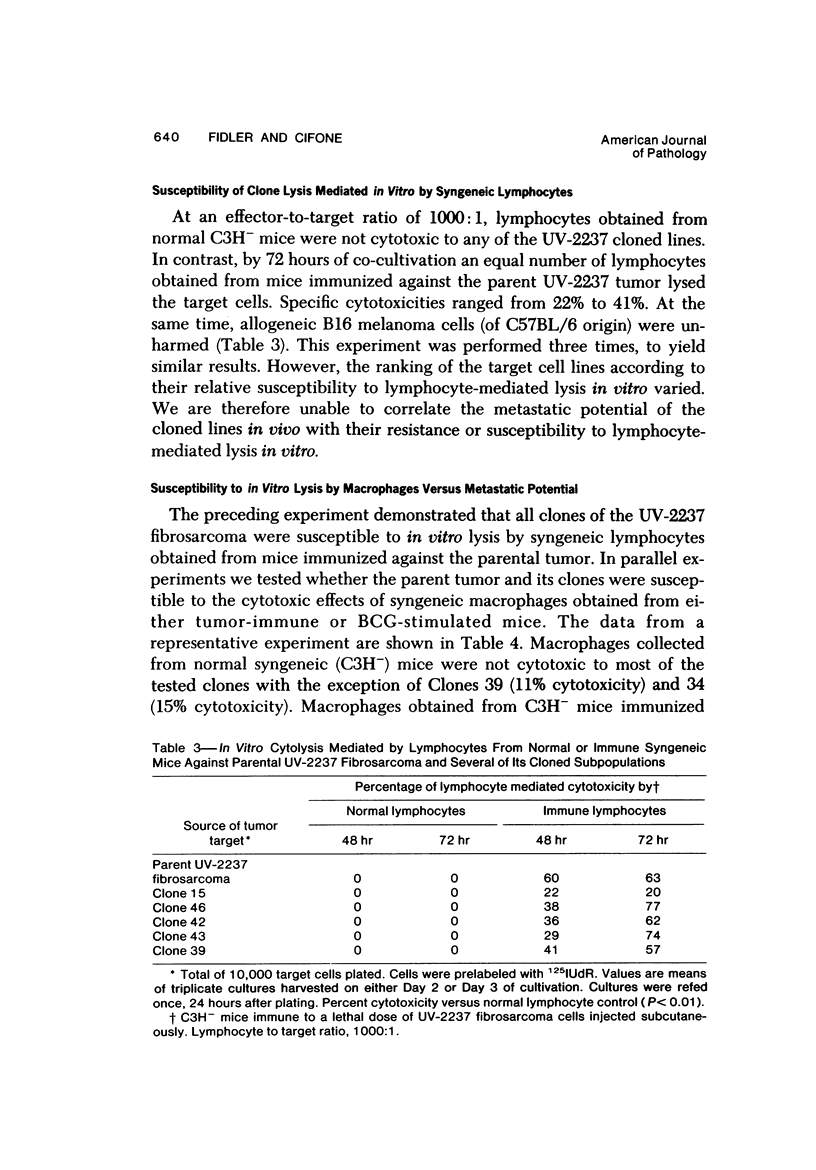
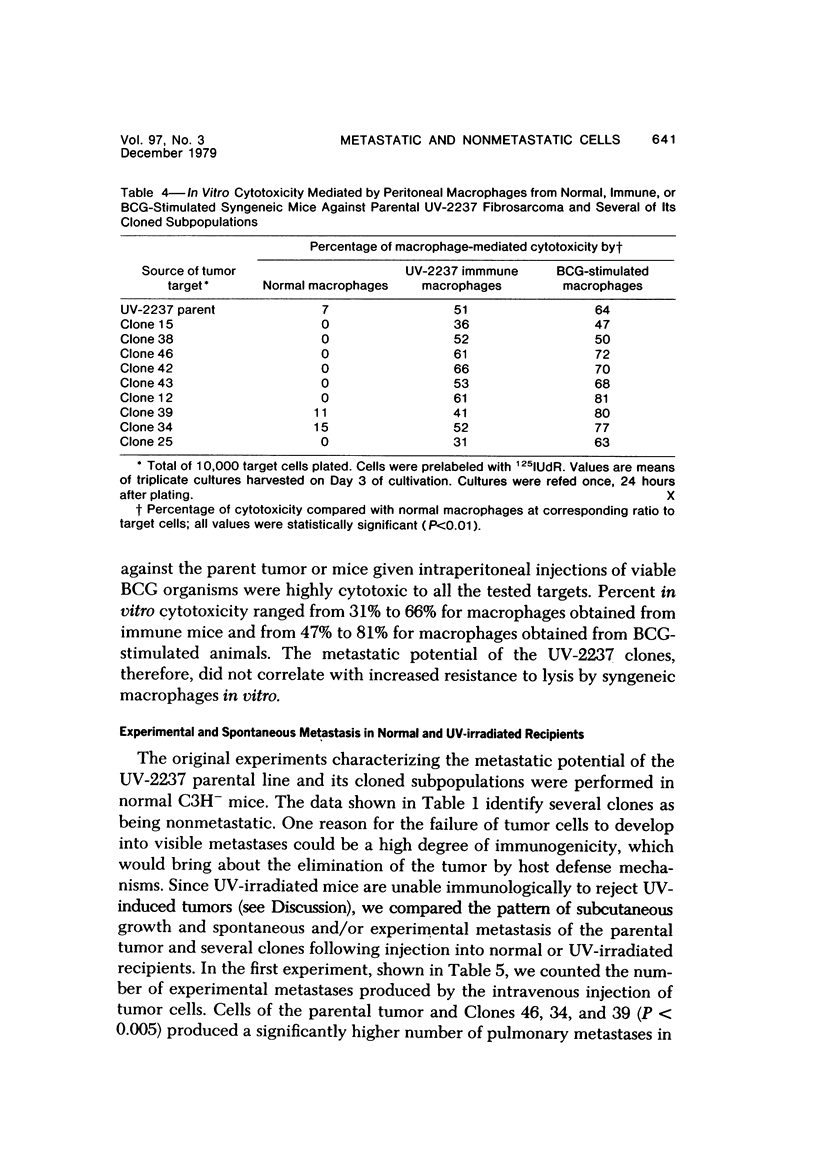
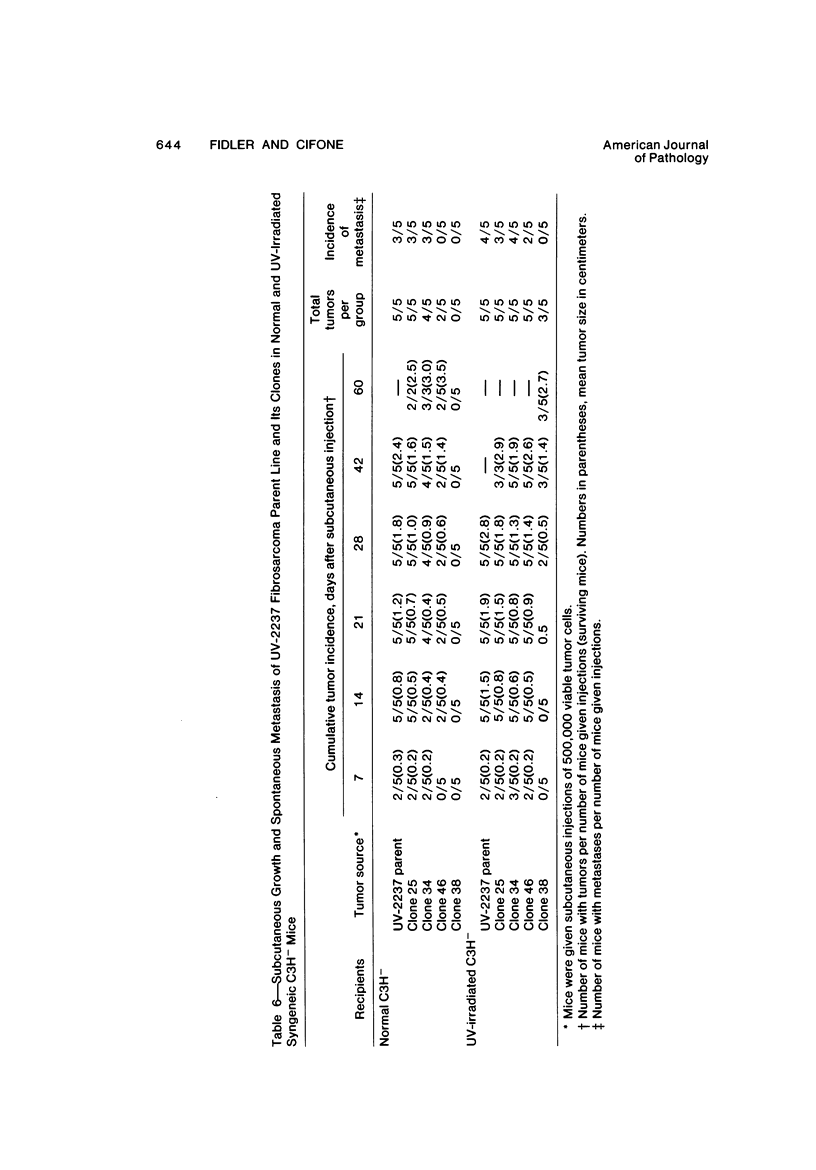
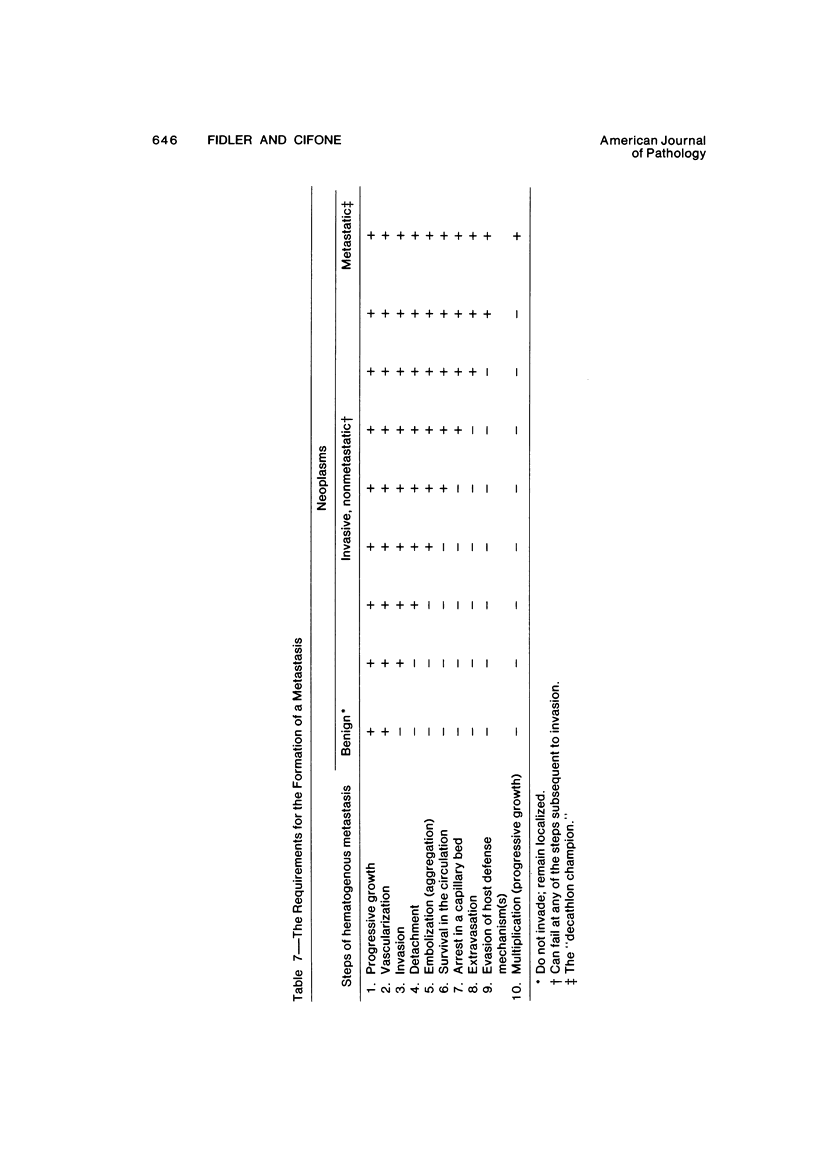
The present studies were designed to evaluate whether tumor cell properties such as growth rate, chromosome number, anchorage-independent growth, susceptibility to lymphocyte- or macrophage-mediated lysis in vitro, and antigenicity in vivo correlated with metastatic potential. A murine fibrosarcoma of recent origin induced in a C3H- mouse by chronic irradiation with ultraviolet light was used. Cells from the parent tumor and its clones were grown in culture. No single property of tumor cells that was measured in vitro or in vivo predicted or correlated with their metastatic potential. In order for metastasis to occur, all steps of the process must be completed. Therefore, interruption of the sequence at any stage can prevent the production of visible metastasis. It was concluded that the search for a single property common to all metastatic cells in a large variety of neoplasms is likely to be unproductive.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colburn N. H., Bruegge W. F., Bates J. R., Gray R. H., Rossen J. D., Kelsey W. H., Shimada T. Correlation of anchorage-independent growth with tumorigenicity of chemically transformed mouse epidermal cells. Cancer Res. 1978 Mar;38(3):624–634. [PubMed] [Google Scholar]
- Deodhar S. D. Enhancement of metastases by L-asparaginase in a mouse tumour system. Nature. 1971 Jun 4;231(5301):319–321. doi: 10.1038/231319a0. [DOI] [PubMed] [Google Scholar]
- Eccles S. A., Alexander P. Immunologically-mediated restraint of latent tumour metastases. Nature. 1975 Sep 4;257(5521):52–53. doi: 10.1038/257052a0. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Darnell J. H., Budmen M. B. Tumoricidal properties of mouse macrophages activated with mediators from rat lymphocytes stimulated with concanavalin A. Cancer Res. 1976 Oct;36(10):3608–3615. [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Hart I. R. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Immune stimulation-inhibition of experimental cancer metastasis. Cancer Res. 1974 Mar;34(3):491–498. [PubMed] [Google Scholar]
- Fidler I. J., Kripke M. L. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977 Aug 26;197(4306):893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Recognition and destruction of target cells by tumoricidal macrophages. Isr J Med Sci. 1978 Jan;14(1):177–191. [PubMed] [Google Scholar]
- Fidler I. J., Roblin R. O., Poste G. In vitro tumoricidal activity of macrophages against virus-transformed lines with temperature-dependent transformed phenotypic characteristics. Cell Immunol. 1978 Jun;38(1):131–146. doi: 10.1016/0008-8749(78)90039-4. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978 Sep;38(9):2651–2660. [PubMed] [Google Scholar]
- Fisher M. S., Kripke M. L. Further studies on the tumor-specific suppressor cells induced by ultraviolet radiation. J Immunol. 1978 Sep;121(3):1139–1144. [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Hewitt H. B., Blake E. R., Walder A. S. A critique of the evidence for active host defence against cancer, based on personal studies of 27 murine tumours of spontaneous origin. Br J Cancer. 1976 Mar;33(3):241–259. doi: 10.1038/bjc.1976.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U., Baumler A., Carruthers C., Bielat K. Immunological escape mechanism in spontaneously metastasizing mammary tumors. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1012–1016. doi: 10.1073/pnas.72.3.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke M. L. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974 Nov;53(5):1333–1336. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- Kripke M. L., Fisher M. S. Immunologic parameters of ultraviolet carcinogenesis. J Natl Cancer Inst. 1976 Jul;57(1):211–215. doi: 10.1093/jnci/57.1.211. [DOI] [PubMed] [Google Scholar]
- Kripke M. L., Gruys E., Fidler I. J. Metastatic heterogeneity of cells from an ultraviolet light-induced murine fibrosarcoma of recent origin. Cancer Res. 1978 Sep;38(9):2962–2967. [PubMed] [Google Scholar]
- Kripke M. L. Latency, histology, and antigenicity of tumors induced by ultraviolet light in three inbred mouse strains. Cancer Res. 1977 May;37(5):1395–1400. [PubMed] [Google Scholar]
- Kripke M. L., Lofgreen J. S., Beard J., Jessup J. M., Fisher M. S. In vivo immune responses of mice during carcinogenesis by ultraviolet irradiation. J Natl Cancer Inst. 1977 Oct;59(4):1227–1230. doi: 10.1093/jnci/59.4.1227. [DOI] [PubMed] [Google Scholar]
- Kripke M. L., Thorn R. M., Lill P. H., Civin C. I., Pazmiño N. H., Fisher M. S. Further characterization of immunological unresponsiveness induced in mice by ultraviolet radiation. Growth and induction of nonultraviolet-induced tumors in ultraviolet-irradiated mice. Transplantation. 1979 Sep;28(3):212–217. doi: 10.1097/00007890-197909000-00012. [DOI] [PubMed] [Google Scholar]
- Norbury K. C., Fidler I. J. In vitro tumor cell destruction by syngeneic mouse macrophoages: methods for assaying cytotoxicity. J Immunol Methods. 1975 Apr;7(1):109–122. doi: 10.1016/0022-1759(75)90136-2. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman C. W., Daynes R. A. Modification of immunological potential by ultraviolet radiation. II. Generation of suppressor cells in short-term UV-irradiated mice. Transplantation. 1977 Aug;24(2):120–126. doi: 10.1097/00007890-197708000-00005. [DOI] [PubMed] [Google Scholar]
- Sugarbaker E. V., Ketcham A. S. Mechanisms and prevention of cancer dissemination: an overview. Semin Oncol. 1977 Mar;4(1):19–32. [PubMed] [Google Scholar]
- Umiel T., Trainin N. Immunological enhancement of tumor growth by syngeneic thymus-derived lymphocytes. Transplantation. 1974 Sep;18(3):244–250. doi: 10.1097/00007890-197409000-00007. [DOI] [PubMed] [Google Scholar]
- Vaage J. A survey of the growth characteristics of and the host reactions to one hundred C3H/He mammary carcinomas. Cancer Res. 1978 Feb;38(2):331–338. [PubMed] [Google Scholar]
- Weiss L. A pathobiologic overview of metastasis. Semin Oncol. 1977 Mar;4(1):5–17. [PubMed] [Google Scholar]


