Abstract
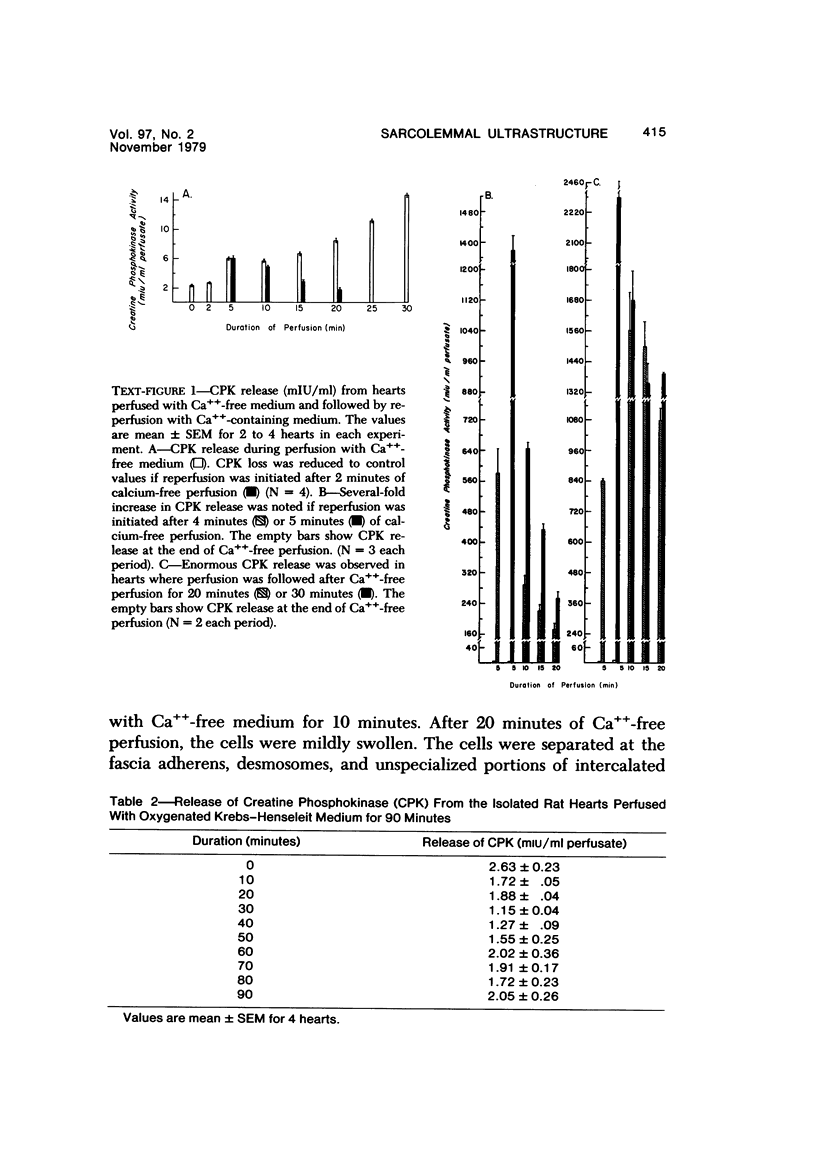
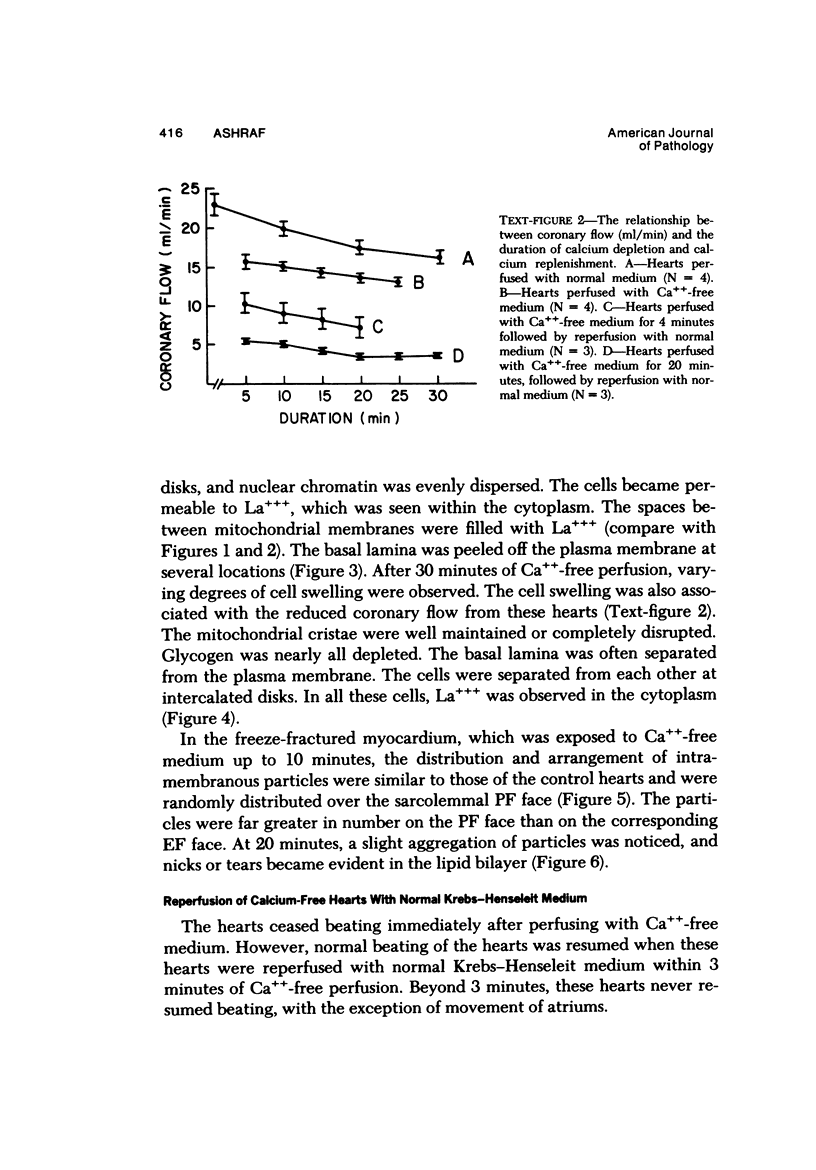
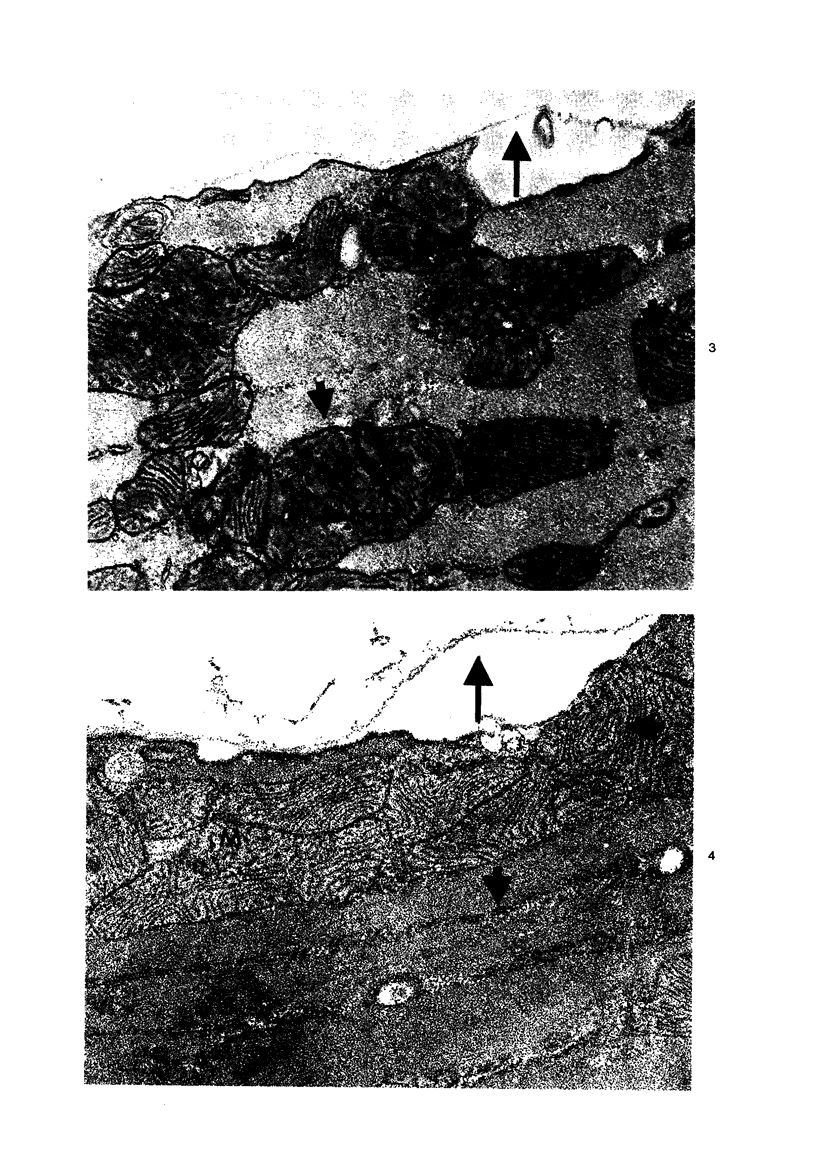
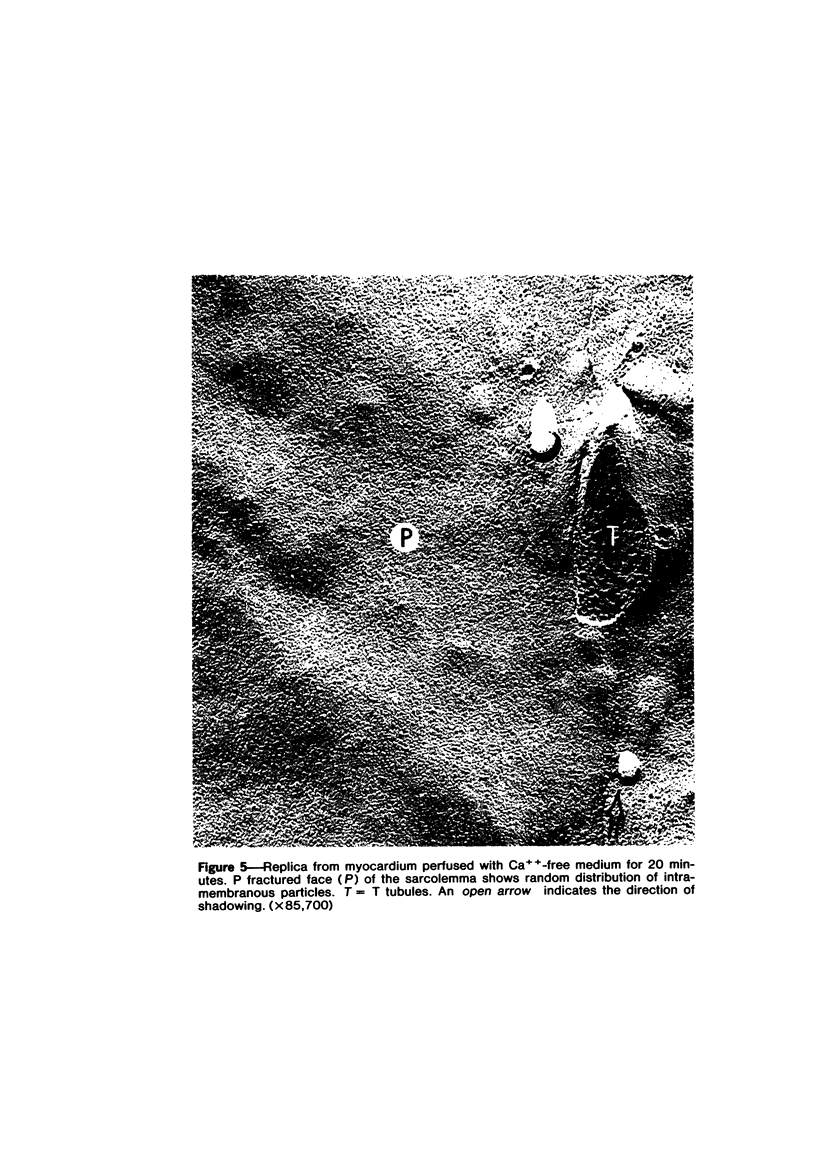
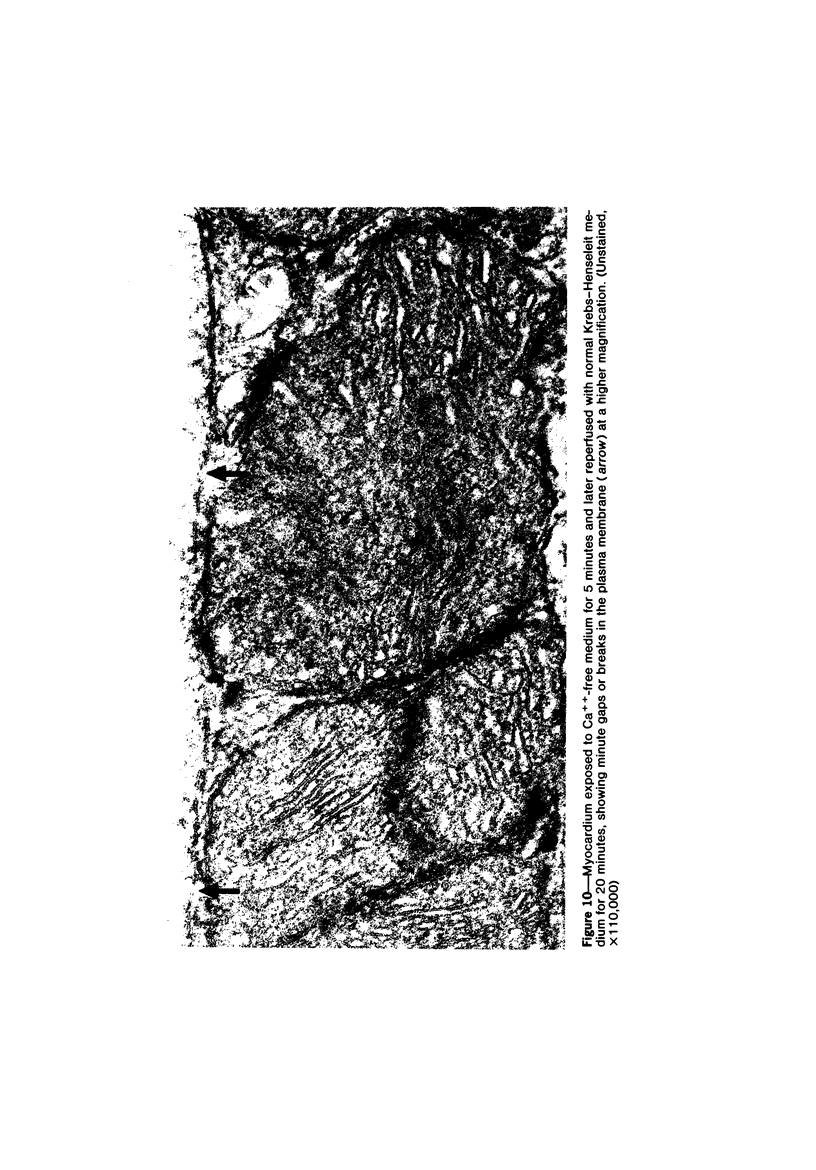
Effects of calcium-free perfusion and calcium-free perfusion followed by reperfusion with calcium on sarcolemmal structure, sarcolemmal permeability, and creatine phosphokinase loss were investigated in isolated perfused rat hearts. Release of creatine phosphokinase was significant (P less than 0.0002) after 4-5 minutes of perfusion with Ca++-free medium, but later releases in comparison to their immediately preceding periods became significant only after more than 20-minute perfusion. Poor correlation between enzyme loss and lanthanum permeability prior to 20 minutes of Ca++-free perfusion was noted. After 20 minutes of Ca++-free perfusion, the basal lamina was separated from the plasma membrane, and lanthanum was seen in the cytoplasm. The intramembranous particles began to aggregate at that time. The morphologic and enzymatic changes were dramatic following reperfusion of calcium-free perfused hearts. Morphologic changes in these hearts included separation of basal lamina, cellular separation at the intercalated disk, dissolution of actin filaments at the region of I band, contraction bands, cell swelling, and staining or filling of mitochondrial membranes with La+++. Increased sarcolemmal permeability was associated with tears and aggregation of intramembranous particles in the sarcolemmal lipid bilayers. These results suggest that reperfusion of Ca++-free perfused cells causes irreversible damage to the sarcolemmal lipid bilayer, and the degree of alterations induced in the cells is dependent upon the initial duration of Ca++-free perfusion.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashraf M., Halverson C. A. Structural changes in the freeze-fractured sarcolemma of ischemic myocardium. Am J Pathol. 1977 Sep;88(3):583–594. [PMC free article] [PubMed] [Google Scholar]
- Bailey L. E., Dresel P. E. Correlation of contractile force with a calcium pool in the isolated cat heart. J Gen Physiol. 1968 Dec;52(6):969–982. doi: 10.1085/jgp.52.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bionk A. B., Ruigrok T. J., Maas A. H., Zimmerman A. N. Changes in high-energy phosphate compounds of isolated rat hearts during Ca2+-free perfusion and reperfusion with Ca2+. J Mol Cell Cardiol. 1976 Dec;8(12):973–979. doi: 10.1016/0022-2828(76)90078-x. [DOI] [PubMed] [Google Scholar]
- Bloor C. M., Ashraf M. Pathogenesis of acute myocardial infarction. Adv Cardiol. 1978;23:1–13. doi: 10.1159/000401068. [DOI] [PubMed] [Google Scholar]
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Bulkley B. H., Nunnally R. L., Hollis D. P. "Calcium paradox" and the effect of varied temperature on its development: a phosphorus nuclear magnetic resonance and morphologic study. Lab Invest. 1978 Aug;39(2):133–140. [PubMed] [Google Scholar]
- De Leiris J., Feuvray D. Factors affecting the release of lactate dehydrogenase from isolated rat heart after calcium and magnesium free perfusions. Cardiovasc Res. 1973 May;7(3):383–390. doi: 10.1093/cvr/7.3.383. [DOI] [PubMed] [Google Scholar]
- Frank J. S., Langer G. A., Nudd L. M., Seraydarian K. The myocardial cell surface, its histochemistry, and the effect of sialic acid and calcium removal on its stucture and cellular ionic exchange. Circ Res. 1977 Nov;41(5):702–714. doi: 10.1161/01.res.41.5.702. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Worstell J., Kaltenbach J. P. Oxygen-induced enzyme release after irreversible myocardial injury. Effects of cyanide in perfused rat hearts. Am J Pathol. 1976 Aug;84(2):327–350. [PMC free article] [PubMed] [Google Scholar]
- Gergely J. Excitation-contraction coupling--cardiac muscle events in the myofilament. Fed Proc. 1976 May 1;35(6):1283–1287. [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Bullock G. R. The oxygen paradox and the calcium paradox: two facets of the same problem? J Mol Cell Cardiol. 1978 Jul;10(7):641–668. doi: 10.1016/s0022-2828(78)80004-2. [DOI] [PubMed] [Google Scholar]
- Holland C. E., Jr, Olson R. E. Prevention by hypothermia of paradoxical calcium necrosis in cardiac muscle. J Mol Cell Cardiol. 1975 Dec;7(12):917–928. doi: 10.1016/0022-2828(75)90152-2. [DOI] [PubMed] [Google Scholar]
- Langer G. A. The structure and function of the myocardial cell surface. Am J Physiol. 1978 Nov;235(5):H461–H468. doi: 10.1152/ajpheart.1978.235.5.H461. [DOI] [PubMed] [Google Scholar]
- McIntyre J. A., Gilula N. B., Karnovsky M. J. Cryoprotectant-induced redistribution of intramembranous particles in mouse lymphocytes. J Cell Biol. 1974 Jan;60(1):192–203. doi: 10.1083/jcb.60.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G., Grau A., Slade A. A protective effect of verapamil on hypoxic heart muscle. Cardiovasc Res. 1976 Nov;10(6):650–662. doi: 10.1093/cvr/10.6.650. [DOI] [PubMed] [Google Scholar]
- Revel J. P., Karnovsky M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967 Jun;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]
- Ruigrok T. J., Boink A. B., Spies F., Blok F. J., Maas A. H., Zimmerman A. N. Energy dependence of the calcium paradox. J Mol Cell Cardiol. 1978 Nov;10(11):991–1002. doi: 10.1016/0022-2828(78)90395-4. [DOI] [PubMed] [Google Scholar]
- Shine K. I., Serena S. D., Langer G. A. Kinetic localization of contractile calcium in rabbit myocardium. Am J Physiol. 1971 Nov;221(5):1408–1417. doi: 10.1152/ajplegacy.1971.221.5.1408. [DOI] [PubMed] [Google Scholar]
- Wade J. B., Kachadorian W. A., DiScala V. A. Freeze-fracture electron microscopy: relationship of membrane structural features to transport physiology. Am J Physiol. 1977 Feb;232(2):F77–F83. doi: 10.1152/ajprenal.1977.232.2.F77. [DOI] [PubMed] [Google Scholar]
- Winegrad S. Studies of cardiac muscle with a high permeability to calcium produced by treatment with ethylenediaminetetraacetic acid. J Gen Physiol. 1971 Jul;58(1):71–93. doi: 10.1085/jgp.58.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. C., Dhalla N. S. Structural and functional changes associated with failure and recovery of hearts after perfusion with Ca2+-free medium. J Mol Cell Cardiol. 1975 Feb;7(2):91–103. doi: 10.1016/0022-2828(75)90011-5. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. N., Hülsmann W. C. Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature. 1966 Aug 6;211(5049):646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]