Abstract
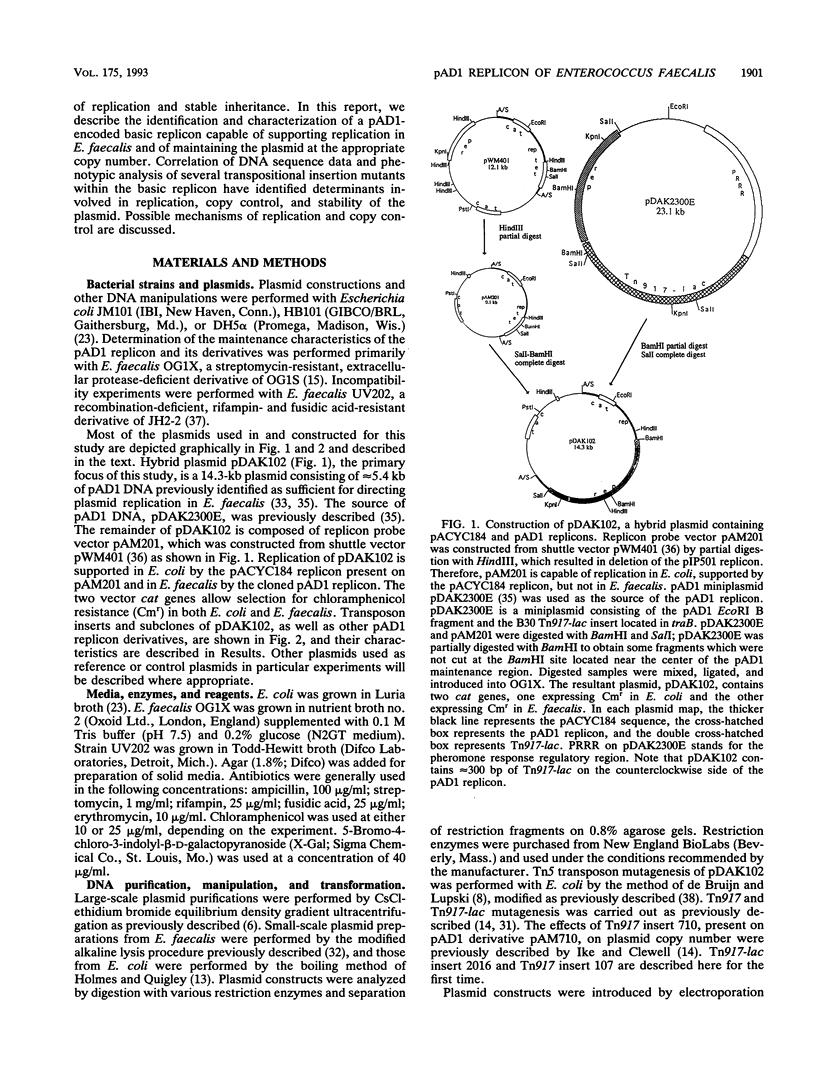
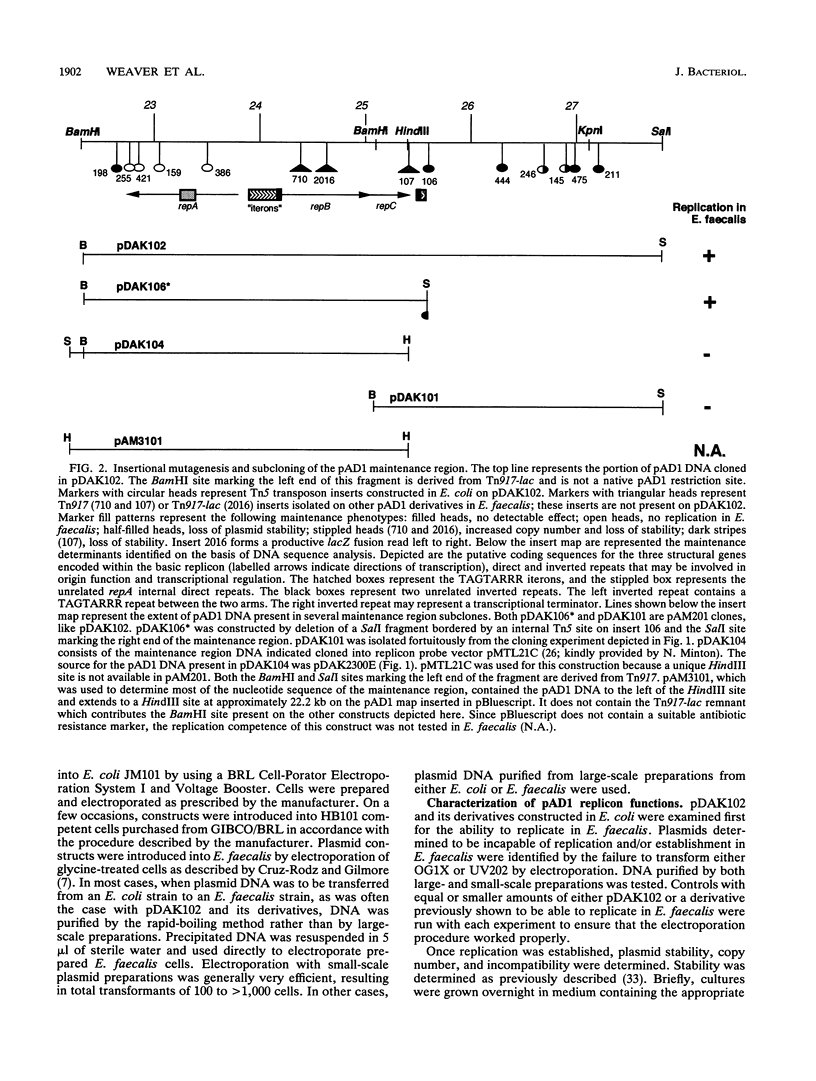
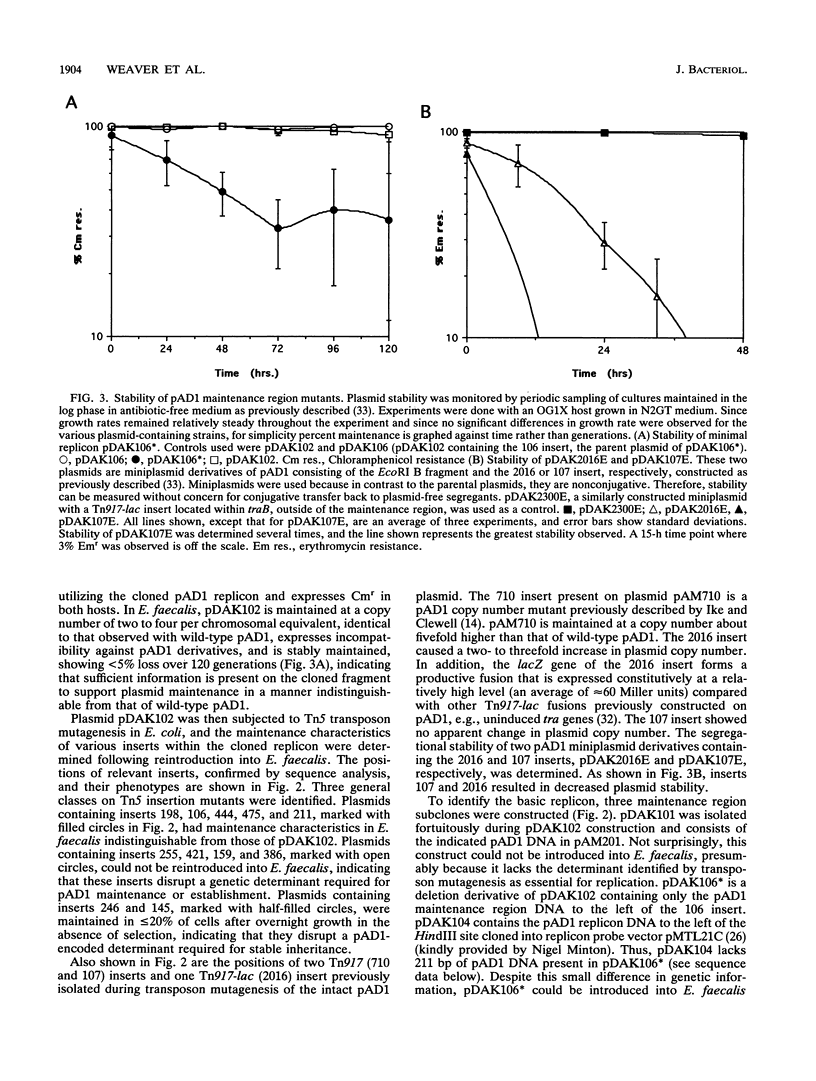
A 5-kbp region of pAD1, previously shown to be capable of supporting replication, copy control, and stable inheritance of the plasmid, was cloned into a replicon probe vector and subjected to transposon insertional mutagenesis. Transposon inserts identifying essential replication, copy control, and stability functions were isolated. Deletion of stability functions not essential for replication resulted in delimitation of a basic replicon. The complete DNA sequence of this approximately 3-kbp region and the precise positions of several transposon inserts were determined, and the phenotypic effects of the transposon inserts were correlated with the physical locations of individual determinants. The following three genes, apparently involved in plasmid maintenance, were identified; repA, which encodes a protein required for replication; repB, which encodes a protein involved in copy control; and repC, which may be involved in stable inheritance. In addition, two clusters of repeats composed of a consensus sequence, TAGTARRR, were identified, one located between the divergently transcribed repA and repB genes and another located downstream of repC. The region between repA and repB contained 25 repeats divided into two subregions of 13 and 12 repeats separated by 78 bp. The region located downstream of repC contained only three repeats but may be essential for plasmid replication, since deletion of this determinant resulted in loss of ability to replicate in Enterococcus faecalis. We hypothesize that the repeat units represent protein-binding sites required for assembly of the replisome and control of plasmid copy number. Another region of unrelated repeat units that may also be involved in replication is located within the repA gene. Possible mechanisms of action of these determinants are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brantl S., Behnke D. Copy number control of the streptococcal plasmid pIP501 occurs at three levels. Nucleic Acids Res. 1992 Feb 11;20(3):395–400. doi: 10.1093/nar/20.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruand C., Ehrlich S. D., Jannière L. Unidirectional theta replication of the structurally stable Enterococcus faecalis plasmid pAM beta 1. EMBO J. 1991 Aug;10(8):2171–2177. doi: 10.1002/j.1460-2075.1991.tb07752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., An F. Y., White B. A., Gawron-Burke C. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918). J Bacteriol. 1985 Jun;162(3):1212–1220. doi: 10.1128/jb.162.3.1212-1220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Weaver K. E. Sex pheromones and plasmid transfer in Enterococcus faecalis. Plasmid. 1989 May;21(3):175–184. doi: 10.1016/0147-619x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Rodz A. L., Gilmore M. S. High efficiency introduction of plasmid DNA into glycine treated Enterococcus faecalis by electroporation. Mol Gen Genet. 1990 Oct;224(1):152–154. doi: 10.1007/BF00259462. [DOI] [PubMed] [Google Scholar]
- Dunny G. M. Genetic functions and cell-cell interactions in the pheromone-inducible plasmid transfer system of Enterococcus faecalis. Mol Microbiol. 1990 May;4(5):689–696. doi: 10.1111/j.1365-2958.1990.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Galli D., Friesenegger A., Wirth R. Transcriptional control of sex-pheromone-inducible genes on plasmid pAD1 of Enterococcus faecalis and sequence analysis of a third structural gene for (pPD1-encoded) aggregation substance. Mol Microbiol. 1992 May;6(10):1297–1308. doi: 10.1111/j.1365-2958.1992.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Gilmore M. S., Segarra R. A., Booth M. C. An HlyB-type function is required for expression of the Enterococcus faecalis hemolysin/bacteriocin. Infect Immun. 1990 Dec;58(12):3914–3923. doi: 10.1128/iai.58.12.3914-3923.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss A., Ehrlich S. D. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989 Jun;53(2):231–241. doi: 10.1128/mr.53.2.231-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Ike Y., Clewell D. B. Genetic analysis of the pAD1 pheromone response in Streptococcus faecalis, using transposon Tn917 as an insertional mutagen. J Bacteriol. 1984 Jun;158(3):777–783. doi: 10.1128/jb.158.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Craig R. A., White B. A., Yagi Y., Clewell D. B. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5369–5373. doi: 10.1073/pnas.80.17.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Hashimoto H., Clewell D. B. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect Immun. 1984 Aug;45(2):528–530. doi: 10.1128/iai.45.2.528-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Hashimoto H., Clewell D. B. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J Clin Microbiol. 1987 Aug;25(8):1524–1528. doi: 10.1128/jcm.25.8.1524-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett B. D., Jensen H. G., Nordquist R. E., Gilmore M. S. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992 Jun;60(6):2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft B., Marre R., Schramm U., Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992 Jan;60(1):25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., An F. Y., Clewell D. B. Plasmids and pheromone response of the beta-lactamase producer Streptococcus (Enterococcus) faecalis HH22. Antimicrob Agents Chemother. 1988 Apr;32(4):547–551. doi: 10.1128/aac.32.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood J. I., Cole S. T. Molecular genetics and pathogenesis of Clostridium perfringens. Microbiol Rev. 1991 Dec;55(4):621–648. doi: 10.1128/mr.55.4.621-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannomiya P., Craig R. A., Clewell D. B., Suzuki A., Fujino M., Till G. O., Marasco W. A. Characterization of a class of nonformylated Enterococcus faecalis-derived neutrophil chemotactic peptides: the sex pheromones. Proc Natl Acad Sci U S A. 1990 Jan;87(1):66–70. doi: 10.1073/pnas.87.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra R. A., Booth M. C., Morales D. A., Huycke M. M., Gilmore M. S. Molecular characterization of the Enterococcus faecalis cytolysin activator. Infect Immun. 1991 Apr;59(4):1239–1246. doi: 10.1128/iai.59.4.1239-1246.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinfield T. J., Oultram J. D., Thompson D. E., Brehm J. K., Minton N. P. Physical characterisation of the replication region of the Streptococcus faecalis plasmid pAM beta 1. Gene. 1990 Mar 1;87(1):79–90. [PubMed] [Google Scholar]
- Tabata S., Hooykaas P. J., Oka A. Sequence determination and characterization of the replicator region in the tumor-inducing plasmid pTiB6S3. J Bacteriol. 1989 Mar;171(3):1665–1672. doi: 10.1128/jb.171.3.1665-1672.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi R., Hashiba H., Aoyama K., Ishii S. Complete nucleotide sequence and characterization of a cryptic plasmid from Lactobacillus helveticus subsp. jugurti. Appl Environ Microbiol. 1989 Jun;55(6):1653–1655. doi: 10.1128/aem.55.6.1653-1655.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Damle S. P., Clewell D. B. Plasmid-related transmissibility and multiple drug resistance in Streptococcus faecalis subsp. zymogenes strain DS16. Antimicrob Agents Chemother. 1979 Jun;15(6):828–830. doi: 10.1128/aac.15.6.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K. E., Clewell D. B. Construction of Enterococcus faecalis pAD1 miniplasmids: identification of a minimal pheromone response regulatory region and evaluation of a novel pheromone-dependent growth inhibition. Plasmid. 1989 Sep;22(2):106–119. doi: 10.1016/0147-619x(89)90020-6. [DOI] [PubMed] [Google Scholar]
- Weaver K. E., Clewell D. B. Control of Enterococcus faecalis sex pheromone cAD1 elaboration: effects of culture aeration and pAD1 plasmid-encoded determinants. Plasmid. 1991 May;25(3):177–189. doi: 10.1016/0147-619x(91)90011-k. [DOI] [PubMed] [Google Scholar]
- Weaver K. E., Clewell D. B. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: construction and characterization of lacZ transcriptional fusions in a key control region of the plasmid. J Bacteriol. 1988 Sep;170(9):4343–4352. doi: 10.1128/jb.170.9.4343-4352.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K. E., Clewell D. B. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: effects of host strain and traA, traB, and C region mutants on expression of an E region pheromone-inducible lacZ fusion. J Bacteriol. 1990 May;172(5):2633–2641. doi: 10.1128/jb.172.5.2633-2641.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y., Clewell D. B. Recombination-deficient mutant of Streptococcus faecalis. J Bacteriol. 1980 Aug;143(2):966–970. doi: 10.1128/jb.143.2.966-970.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Jones J. M., Senghas E., Gawron-Burke C., Clewell D. B. Generation of Tn5 insertions in streptococcal conjugative transposon Tn916. Appl Environ Microbiol. 1987 May;53(5):1069–1072. doi: 10.1128/aem.53.5.1069-1072.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Tamm I. Molecular cloning and characterization of interferon alpha/beta response element binding factors of the murine (2'-5')oligoadenylate synthetase ME-12 gene. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):144–148. doi: 10.1073/pnas.88.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]