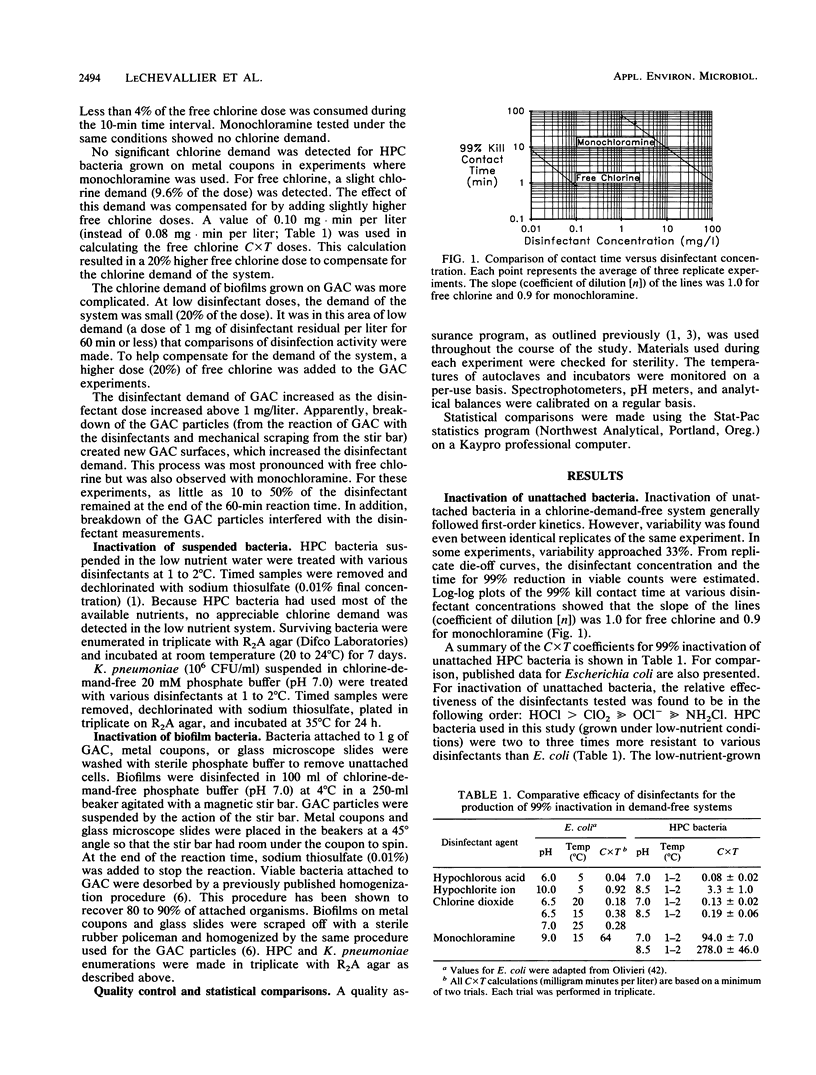
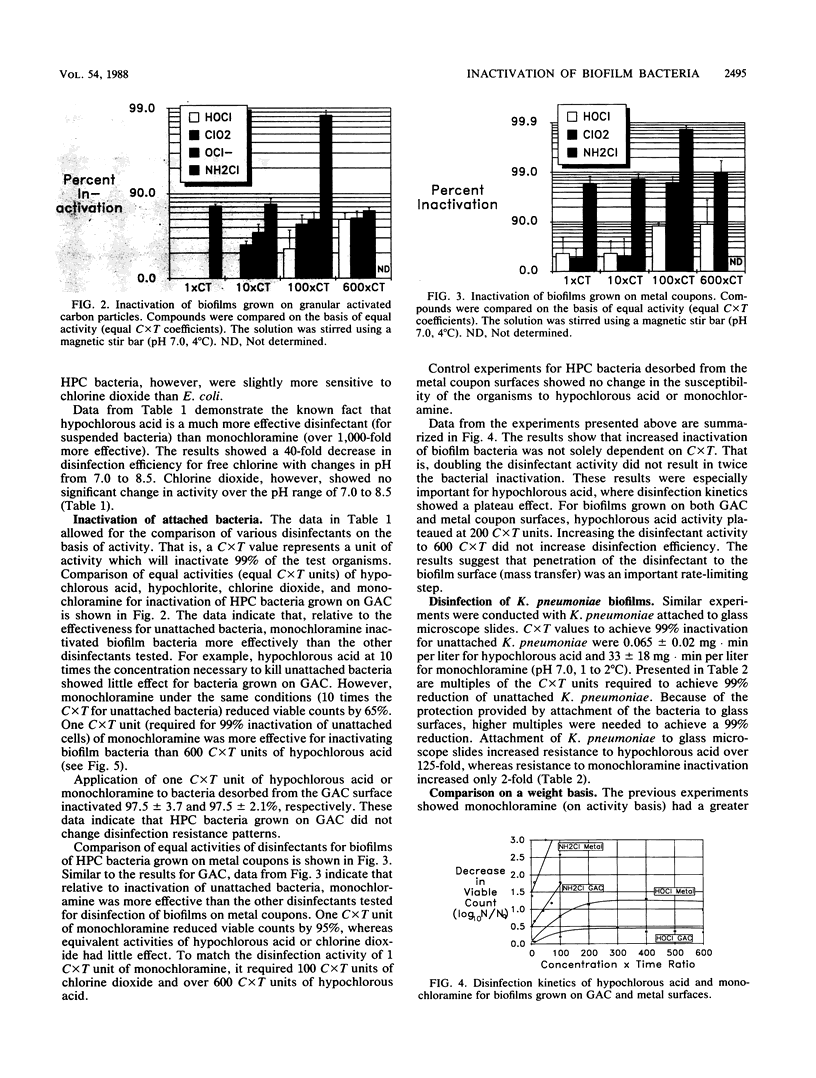
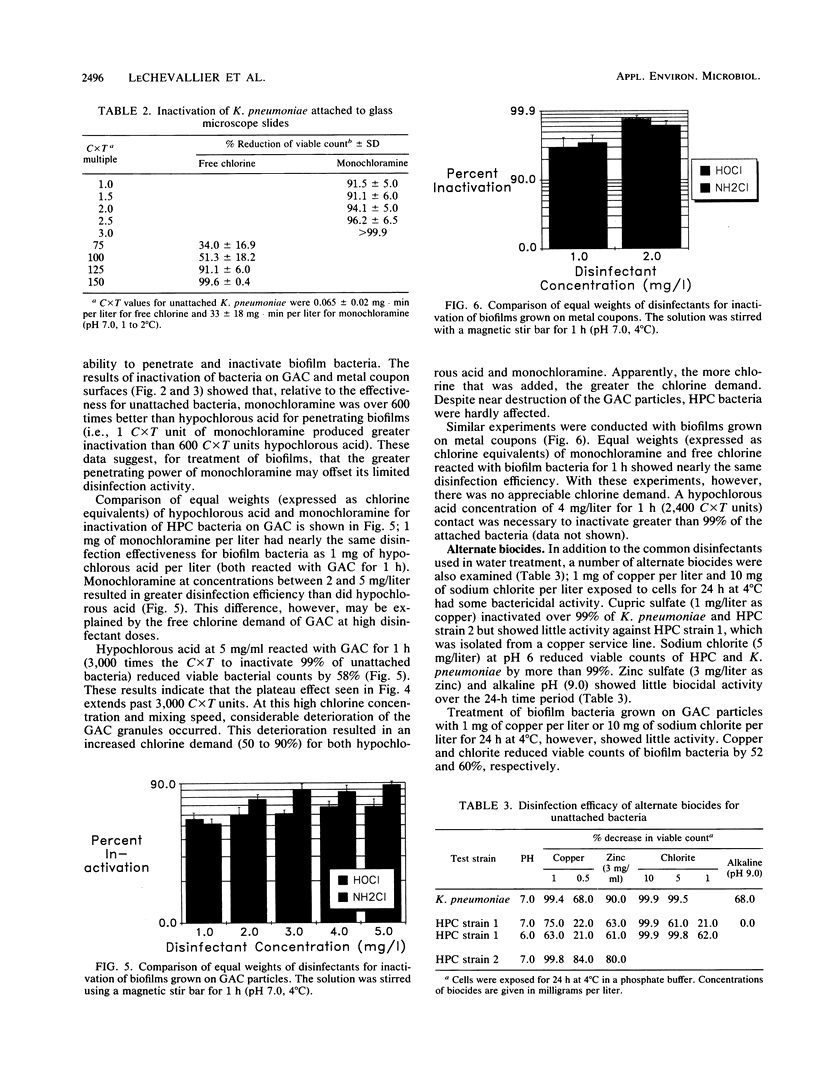
Abstract
The current project was developed to examine inactivation of biofilm bacteria and to characterize the interaction of biocides with pipe surfaces. Unattached bacteria were quite susceptible to the variety of disinfectants tested. Viable bacterial counts were reduced 99% by exposure to 0.08 mg of hypochlorous acid (pH 7.0) per liter (1 to 2 degrees C) for 1 min. For monochloramine, 94 mg/liter was required to kill 99% of the bacteria within 1 min. These results were consistent with those found by other investigators. Biofilm bacteria grown on the surfaces of granular activated carbon particles, metal coupons, or glass microscope slides were 150 to more than 3,000 times more resistant to hypochlorous acid (free chlorine, pH 7.0) than were unattached cells. In contrast, resistance of biofilm bacteria to monochloramine disinfection ranged from 2- to 100-fold more than that of unattached cells. The results suggested that, relative to inactivation of unattached bacteria, monochloramine was better able to penetrate and kill biofilm bacteria than free chlorine. For free chlorine, the data indicated that transport of the disinfectant into the biofilm was a major rate-limiting factor. Because of this phenomenon, increasing the level of free chlorine did not increase disinfection efficiency. Experiments where equal weights of disinfectants were used suggested that the greater penetrating power of monochloramine compensated for its limited disinfection activity. These studies showed that monochloramine was as effective as free chlorine for inactivation of biofilm bacteria. The research provides important insights into strategies for control of biofilm bacteria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. L., Calomiris J. J., Seidler R. J. Selection of antibiotic-resistant standard plate count bacteria during water treatment. Appl Environ Microbiol. 1982 Aug;44(2):308–316. doi: 10.1128/aem.44.2.308-316.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper A. K., LeChevallier M. W., Broadaway S. C., McFeters G. A. Bacteria associated with granular activated carbon particles in drinking water. Appl Environ Microbiol. 1986 Sep;52(3):434–438. doi: 10.1128/aem.52.3.434-438.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domek M. J., LeChevallier M. W., Cameron S. C., McFeters G. A. Evidence for the role of copper in the injury process of coliform bacteria in drinking water. Appl Environ Microbiol. 1984 Aug;48(2):289–293. doi: 10.1128/aem.48.2.289-293.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejkal T. W., Wellings F. M., LaRock P. A., Lewis A. L. Survival of poliovirus within organic solids during chlorination. Appl Environ Microbiol. 1979 Jul;38(1):114–118. doi: 10.1128/aem.38.1.114-118.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson D. S., McGonigle B., Payer M. A., Baker K. H. Attachment as a factor in the protection of Enterobacter cloacae from chlorination. Appl Environ Microbiol. 1987 May;53(5):1178–1180. doi: 10.1128/aem.53.5.1178-1180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChevallier M. W., Babcock T. M., Lee R. G. Examination and characterization of distribution system biofilms. Appl Environ Microbiol. 1987 Dec;53(12):2714–2724. doi: 10.1128/aem.53.12.2714-2724.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChevallier M. W., Cawthon C. D., Lee R. G. Factors promoting survival of bacteria in chlorinated water supplies. Appl Environ Microbiol. 1988 Mar;54(3):649–654. doi: 10.1128/aem.54.3.649-654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChevallier M. W., Evans T. M., Seidler R. J. Effect of turbidity on chlorination efficiency and bacterial persistence in drinking water. Appl Environ Microbiol. 1981 Jul;42(1):159–167. doi: 10.1128/aem.42.1.159-167.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChevallier M. W., Hassenauer T. S., Camper A. K., McFeters G. A. Disinfection of bacteria attached to granular activated carbon. Appl Environ Microbiol. 1984 Nov;48(5):918–923. doi: 10.1128/aem.48.5.918-923.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc H., Mizon F. Eaux d'alimentation et bactéries résistantes aux antibiotiques. Incidences sur les normes. Rev Epidemiol Sante Publique. 1978;26(2):137–146. [PubMed] [Google Scholar]
- Levy R. V., Cheetham R. D., Davis J., Winer G., Hart F. L. Novel method for studying the public health significance of macroinvertebrates occurring in potable water. Appl Environ Microbiol. 1984 May;47(5):889–894. doi: 10.1128/aem.47.5.889-894.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway H. F., Olson B. H. Chlorine resistance patterns of bacteria from two drinking water distribution systems. Appl Environ Microbiol. 1982 Oct;44(4):972–987. doi: 10.1128/aem.44.4.972-987.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Morrow J. E., Bagley S. T. Klebsielleae in drinking water emanating from redwood tanks. Appl Environ Microbiol. 1977 Apr;33(4):893–900. doi: 10.1128/aem.33.4.893-900.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]