Abstract
Background and Purpose:
Cannabinoids are associated with analgesia in acute and chronic pain states. A spectrum of central cannabinoid (CB1) receptor-mediated motor and psychotropic side effects limit their therapeutic potential. Here, we investigate the analgesic effect of the palmitoylethanolamide (PEA) analogue, palmitoylallylamide (L-29), which via inhibition of fatty acid amide hydrolase (FAAH) may potentiate endocannabinoids thereby avoiding psychotropic side effects.
Experimental Approach:
The in vivo analysis of the effect of L-29 on measures of pain behaviour in three rat models of neuropathic pain.
Key Results:
Systemically administered L-29 (10 mg kg−1) reduced hypersensitivity to mechanical and thermal stimuli in the partial sciatic nerve injury (PSNI) model of neuropathic pain; and mechanical hypersensitivity in a model of antiretroviral (ddC)-associated hypersensitivity and a model of varicella zoster virus (VZV)-associated hypersensitivity. The effects of L-29 were comparable to those of gabapentin (50 mg kg−1). The CB1 receptor antagonist SR141716a (1 mg kg−1) and the CB2 receptor antagonist SR144528 (1 mg kg−1) reduced the effect of L-29 on hypersensitivity in the PSNI and ddC models, but not in the VZV model. The peroxisome proliferator-activated receptor-α antagonist, MK-886 (1 mg kg−1), partially attenuated the effect of L-29 on hypersensitivity in the PSNI model. L-29 (10 mg kg−1) significantly attenuated thigmotactic behaviour in the open field arena without effect on locomotor activity.
Conclusions and Implications:
L-29 produces analgesia in a range of neuropathic pain models. This presents L-29 as a novel analgesic compound that may target the endogenous cannabinoid system while avoiding undesirable side effects associated with direct cannabinoid receptor activation.
Keywords: neuropathic pain, cannabinoids, palmitoylethanolamide, palmitoylallylamide, FAAH, open field activity
Introduction
There is now considerable evidence demonstrating that Δ9-tetrahydrocannabinol (THC), the psychoactive ingredient of Cannabis sativa, and a number of synthetic cannabinoid (CB) receptor agonists have analgesic activity in rodent models of acute nociception and persistent pain (Rice, 2005) and also clinical efficacy in central neuropathic pain associated with multiple sclerosis (Rice et al., 2007). In particular, cannabinoid agonists attenuate signs of hindpaw hypersensitivity associated with nerve injury-induced models of neuropathic pain (Herzberg et al., 1997; Bridges et al., 2001; Fox et al., 2001; Scott et al., 2004), demyelination-associated pain (Wallace et al., 2003) and with inflammatory pain models (Smith et al., 1998; Farquhar-Smith et al., 2002; Kehl et al., 2003; De Vry et al., 2004). These effects, together with similar findings in models of inflammatory pain, are mediated via both cannabinoid CB1 and CB2 receptors (Bridges et al., 2001; Fox et al., 2001; Farquhar-Smith et al., 2002). However, there is a need to improve the therapeutic index of existing cannabinoids, particularly with respect to short-term motor and psychotropic side effects and the longer-term risk of psychosis associated with Cannabis use, which are mediated by cannabinoid CB1 receptors expressed in brain (Rice et al., 2007).
An alternative approach that may avoid such side effects is to manipulate the endogenous cannabinoid system (Pertwee, 2001; Rice, 2001; Rice et al., 2002; Hohmann and Suplita, 2006). The endogenous cannabinoids (endocannabinoids) include arachidonoyl ethanolamide (anandamide) and 2-arachidonoyl glycerol (2-AG). Anandamide, acting preferentially via the CB1 receptor (Calignano et al., 1998, 2001; Conti et al., 2002; Farquhar-Smith et al., 2002; Farquhar-Smith and Rice, 2003) has been shown to produce analgesia in a number of acute and inflammatory pain models (Smith et al., 1994; Jaggar et al., 1998; Richardson et al., 1998; Farquhar-Smith and Rice, 2001). However, systemically administered endocannabinoids such as anandamide have a reduced efficacy compared to synthetic CB receptor agonists due to their rapid degradation (Smith et al., 1994). The pharmacological actions of anandamide are terminated by its metabolism to arachidonic acid, catalysed by fatty acid amide hydrolase (FAAH) (Giang and Cravatt, 1997; Cravatt et al., 2001). FAAH is also involved in the degradation of 2-AG (de Lago et al., 2005; Jhaveri et al., 2006; Maione et al., 2006) and is one of the two enzymes that metabolises another fatty acid amide, palmitoylethanolamide (PEA), a shorter, fully saturated analogue of anandamide (Lambert et al., 2002; Darmani et al., 2005). PEA is also degraded by a second enzyme, N-acetylethanolamine acid hydrolase (NAAA) (Tsuboi et al., 2005). While FAAH has been shown to degrade 2-AG, the major catabolizing enzyme in vivo is monoacylglycerol lipase, which is also a therapeutic target in a similar fashion to FAAH (Hohmann, 2007).
PEA has been sold as an orally administered anti-inflammatory for decades (LoVerme et al., 2005a) and limited evidence from an unpublished clinical trial suggests that it has efficacy in the neuropathic pain condition of sciatica (Rice, 2001). PEA is efficacious in animal models of inflammatory pain (Calignano et al., 1998, 2001; Jaggar et al., 1998; Farquhar-Smith and Rice, 2001, 2003; Farquhar-Smith et al., 2002), neuropathic pain (Helyes et al., 2003) and spasticity and tremor in multiple sclerosis (Baker et al., 2001). Moreover, endogenous levels of PEA decrease in a model of neuropathic pain (Petrosino et al., 2007).The mechanism by which PEA acts remains something of a mystery. Although the analgesic actions of PEA are consistently antagonised by the CB2 receptor antagonist SR144528 (Calignano et al., 1998, 2001; Farquhar-Smith et al., 2002), it does not have high affinity for CB2 receptors. Several different scenarios of the mechanism of PEA action have been suggested (LoVerme et al., 2005a) including; interaction with hitherto uncharacterised CB2-like receptors at which SR144528 is also a functional antagonist, interaction with the peroxisome proliferator-activated receptor-α (PPAR-α) (LoVerme et al., 2005b) and inhibition of FAAH, thus increasing local concentrations of anandamide and perhaps PEA (the so-called ‘entourage effect') (Ben Shabat et al., 1998; Lambert and Di Marzo, 1999; Lambert et al., 2002; LoVerme et al., 2005b).
Inhibition of FAAH should therefore prolong the actions of anandamide, 2-AG and PEA. Importantly, this effect should be specific to pathways in which synthesis is upregulated as a result of activity, such as in nociceptive pathways in chronic pain conditions. In support of this, mice lacking the faah gene are hypoalgesic, have increased anandamide and PEA concentrations in the brain and display an increase in anandamide-induced analgesia (Cravatt et al., 2001; Lichtman et al., 2004b; Patel et al., 2005). Additionally, the FAAH inhibiting compounds, OL135 and URB597 ameliorate pain behaviours in selected models of inflammatory and/or neuropathic pain in rats (Lichtman et al., 2004b; Leung et al., 2005; Chang et al., 2006; Jayamanne et al., 2006; Jhaveri et al., 2006). However, the exact mechanism by which FAAH inhibition exerts these effects is not clear.
The PEA analogue, palmitoylallylamide (L-29) (Vandevoorde et al., 2003a) does not significantly bind to either CB1 or CB2 receptors and acts as an inhibitor of FAAH thereby blocking the metabolism of anandamide (Vandevoorde et al., 2003a). Therefore, we have investigated the analgesic actions of L-29 in three models of neuropathic pain including, a model of nerve injury-induced pain involving partial sciatic nerve injury (PSNI) (Seltzer et al., 1990), a model of drug-induced neuropathy in treatment of infection with human immunodeficiency virus (HIV) (Joseph et al., 2004; Wallace et al., 2006) and a model of varicella zoster virus (VZV)-associated pain (Garry et al., 2005; Hasnie et al., 2007). The latter models represent more recently characterised, clinically relevant rodent models of persistent pain. We have demonstrated that L-29 (10 mg kg−1) attenuated measures of hypersensitivity in all three models and that CB1 and CB2 receptors were differentially involved in the actions of L-29 between the models and modalities tested. The PPAR-α antagonist reduced the effect of L-29 in the PSNI model, suggesting that it acts, at least partly, via this mechanism. Additionally, L-29 (10 mg kg−1) was effective in reversing measures of anxiety-like behaviour in PSNI-treated rats when assessed in the open field paradigm, a novel integrative measure of pain-associated behaviour. Our results indicate that L-29 may prove to be a useful analgesic drug that potentially avoids the psychotropic side effects of drugs that directly activate CB receptors.
Methods
Animals and surgery
All experiments conformed to the British Home Office Regulations and IASP guidelines (Zimmermann, 1983). Male Wistar rats weighing 200-250 g were used for all experiments (B & K, Hull, UK) and were housed in a temperature-controlled environment, maintained on a 14:10 h light–dark cycle (experiments were performed during the light phase) and provided with feed and water ad libitum.
Nerve injury model
Under 1–2% isoflurane anaesthesia (Abbott, Queenborough, UK) in O2 and N2O, and aseptic surgical conditions, the left sciatic nerve was exposed in the popliteal fossa. A PSNI (Seltzer et al., 1990) was performed in which 1/3–1/2 of the sciatic nerve was tightly ligated using 7.0 suture thread (Ethicon, Edinburgh, UK). The wound was then closed with 4.0 sutures and animals allowed to recover. For sham animals, the same surgical procedure was followed as for PSNI animals, but the nerve was not ligated.
Drug-induced neuropathy model
Animals were injected intraperitoneally (i.p.) with dideoxycitadine (ddC) (50 mg kg−1 in 0.5 ml saline; Sigma-Aldrich, Poole, UK) three times a week for up to 3 weeks (Mon, Wed, Fri) (Joseph et al., 2004; Wallace et al., 2006).
Varicella zoster-induced neuropathy model
This model is based upon the injection of VZV into the hindpaw of a rat (Fleetwood-Walker et al., 1999; Dalziel et al., 2004; Garry et al., 2005; Hasnie et al., 2007). Primary human embryonic lung cells (gift from J Breuer, Royal London Hospital, UK) were inoculated with VZV and harvested when cells exhibited approximately 80% cytopathic effect on microscopy (equivalent to 104–105 PFU). Virus-infected cells were gently scraped from the flask surface onto which they had formed a monolayer culture and the cell suspension centrifuged at 200 g in 4°C for 15 min. The resulting pellet from each 75 cm2 flask was re-suspended in 150 μl sterile phosphate buffer solution (Invitrogen, Paisley, UK). Animals were anaesthetised with pentobarbitone (40 mg kg−1 i.p. Animalcare Ltd., York, UK) and subcutaneously injected with 50 μl viral inoculum into the left (ipsilateral) hind footpad using a 25-gauge needle.
Behavioural reflex testing
The threshold for hindpaw withdrawal in response to graded mechanical stimulation was measured in conscious animals using an electronic ‘von Frey' device (Moller et al., 1998; Ahmad and Rice, 1999) of 0.5 mm2 probe tip area (Somedic Sales AB, Sweden) applied manually at a rate of 8–15 g s−1 to the mid-plantar surface of the hindpaw with the withdrawal threshold (grams) defined as the average force that evoked an active limb withdrawal response over five applications. We have previously validated this as a reliable measure for measuring mechanical hypersensitivity in rodent models of neuropathic pain (Hasnie et al., 2007; Wallace et al., 2007). The time for hindpaw withdrawal in response to a quantified noxious heat stimulus was assessed using the plantar test (Ugo Basile, Comerio, Italy) (Hargreaves et al., 1988). The thermal stimulus (set at an infrared intensity that produced standard latency of approximately 10 s) was applied to the mid-plantar surface of the hindpaw and the latency (seconds) to withdrawal recorded over three applications. The presence of a behavioural correlate of cold allodynia was assessed using the acetone drop application technique (Bridges et al., 2001). A single drop of acetone was applied via a 1 ml syringe to the mid-plantar surface of each hindpaw and the outcome defined as the percentage of applications, which evoked an active limb withdrawal from a total of five applications. The threshold value at each time point tested was calculated as the mean±s.e.m.
Baseline measurements were obtained for all animals over the course of a week before surgery. Animals were then tested on day 7 (for PSNI), or day 14 (for VZV) following surgery; or day 19 following initial ddC injection to identify the development of any reflex sensitivity in treated animals. For PSNI, only animals that developed significant hypersensitivity in all three modalities (at least 30% change from baseline for thermal and mechanical and at least 80% response to acetone) were included in the study. For VZV and ddC models, only animals that developed significant hypersensitivity to mechanical stimulation (at least 30% change from baseline) were included in the study. Drugs were administered over the course of a week and no animals were tested on 2 consecutive days. On each day of drug testing, animals were tested once for each modality (displayed as t=0) to ensure presence of behavioural reflex hypersensitivity before drug injection. All testing was performed by an investigator, unaware of the treatments.
Open field activity
At day 14 post-surgery, rats (n=11 per group) were placed into a 1 × 1 m arena illuminated to 4 lux with a defined inner zone of 40 × 40 cm. Locomotion of the rats within the arena was tracked over a 15 min period recorded using a Sanyo VCB 3372 high-resolution monochrome camera (Tracksys, Notts, UK) and stored and analysed with Ethovision software version 3 (Tracksys). The total distance moved, time spent in the inner zone and the number of entries into the inner zone were calculated and displayed as the mean±s.e.m. (Hasnie et al., 2007; Wallace et al., 2007). Only those with mechanical and thermal hypersensitivity (a change of at least 30% from baseline) were included (100% inclusion rate).
Statistics
For determination of the development of significant behavioural hypersensitivity (P<0.05), a paired t-test was used to compare pre- and post-treatment thermal and mechanical values and a Mann Whitney-Rank Sum test for cold values. To determine significant drug effects, a one-way analysis of variance (ANOVA) with Bonferroni post hoc analysis was used between groups at each time point and a one-way ANOVA with Dunnett's multiple comparisons vs control post hoc analysis for comparing pre- and post-injection threshold values.
Drugs
L-29, SR141716a, SR144528 (NIMH, Bethesda, MD, USA) and MK-886 (Biomol International, Exeter, UK) were all dissolved in a 1:2 mixture of ethanol (absolute molecular grade; VWR, Poole) and cremophor EL (Univar; Essex, UK). For reflex behavioural tests, L-29 was used at doses of 1, 5, 10 and 20 mg kg−1 and injected (i.p. at a volume of 0.15 ml), following the behaviour measure taken at t=0. For receptor antagonist studies, SR141716a (1 mg kg−1 in 0.1 ml) or SR144528 (1 mg kg−1 in 0.1 ml) or MK-886 (1 mg kg−1 in 0.1 ml) were injected 2 min before L-29. Gabapentin (Sigma-Aldrich) was dissolved in saline and injected (50 mg kg−1 in 0.5 ml) at t=0. Behavioural reflex tests were carried out at 20, 40, 60, 80 and 100 min post-injection in a rotational manner. For open field activity, L-29 was used at 10 mg kg−1 and injected (i.p. at a volume of 0.15 ml) 20 min before testing. Animals were randomised as to which treatment they received using randomisation tables and all experiments were conducted without knowledge of drug treatments.
Results
L-29 significantly attenuated mechanical and thermal, but not cold hypersensitivity in PSNI-treated animals
By post-operative day 7, 80% of rats that had undergone PSNI surgery developed significant (P<0.01) sensitivity to (a) thermal stimuli (baseline=11.4±1.5 s vs day 7=6.9±0.9 s) (b) mechanical stimuli (baseline=55.72±7.1 g vs day 7=19.6±3.5 g) and cold stimuli (baseline=7.5±5.5% vs day 7=90±6.1%) ipsilateral to injury and were therefore included in the study. In the drug paradigm, vehicle control-treated animals showed no significant change in paw withdrawal threshold in any modality as compared to pre-injection values. At doses of 1 and 5 mg kg−1, L-29 had no significant effect on the threshold for hindpaw withdrawal from thermal (Figure 1a), mechanical (Figure 1b) or cold stimuli (Figure 1c) as compared to vehicle control. At 10 mg kg−1, there was a significant (P<0.01) attenuation of hypersensitivity to thermal stimuli at 40, 60, 80 and 100 min post-injection as compared to vehicle control and pre-injection values (Figures 1a). There was also a significant (P<0.01) attenuation of hypersensitivity to mechanical stimuli 20, 40, 60 and 100 min post-injection as compared to vehicle control and at 20, 40, 60 and 100 min post-injection as compared to pre-injection values (Figure 1b). At 20 mg kg−1, there was a significant (P<0.01) attenuation of hypersensitivity to thermal stimuli at 40, 80 and 100 min post-injection as compared to vehicle control and pre-injection threshold (Figure 1a). However, there was no significant effect on thresholds to mechanical stimuli as compared to vehicle over the test period (Figure 1b).
Figure 1.
The effect of L-29 (1–20 mg kg−1) on hindpaw reflex behaviours in PSNI-treated rats. Hindpaw withdrawal thresholds to (a) thermal (b) mechanical and (c) a cooling stimulus in PSNI rats treated with L-29 (1–20 mg kg−1) vs vehicle control (n=12 per group). Statistical significance of differences between each dose and the vehicle control (*P<0.01) was determined by a one-way ANOVA with Dunn's all pairwise multiple comparisons or (§P<0.01) between each post-injection value vs the pre-injection values (at time 0) using a one way ANOVA with Dunnett's multiple comparisons vs control post hoc analysis. Each value is the mean±s.e.m. ANOVA, analysis of variance; PSNI, partial sciatic nerve injury.
In all cases, the effect of L-29 at 20 mg kg−1 was less than that of 10 mg kg−1. Therefore, we tested no higher doses of L-29. For all doses tested, there was no significant difference in the paw withdrawal threshold to cold stimuli as compared to vehicle control (Figure 1c) and therefore, we conducted no further investigations using the cold stimulus. The dose response of L-29 on paw withdrawal thresholds to thermal (Figure 2a), mechanical (Figure 2b) and cold (Figure 2c) stimuli was calculated as the % change in paw withdrawal threshold from the pre-injection value (t=0) at the time point at which there was the greatest significant change. This confirmed 10 mg kg−1 to be the optimum dose for further studies of the effect of L-29 on mechanical (a change of 66.6% from pre-injection value at 20 min post-injection) and thermal (a change of 26.5% from pre-injection value at 40 min post-injection) hypersensitivity.
Figure 2.
The dose response of L-29 on hindpaw reflex behaviours in partial sciatic nerve injury-treated rats. Dose response curves for the effect of L-29 on hindpaw withdrawal responses to (a) thermal (at 40 min post-injection), (b) mechanical (at 20 min post-injection) and (c) a cold (40 min post-injection) stimulus. The time point with the largest effect of L-29 for each modality was chosen and values determined as % change from pre-injection value. In all modalities, 10 mg kg−1 is the optimum dose.
L-29 (10 mg kg−1) significantly attenuated mechanical hypersensitivity in ddC-treated animals
In line with previous studies (Wallace et al., 2006), by day 19 after the initial ddC injection, 100% of rats developed a significant (P<0.01) bilateral hindpaw withdrawal sensitivity to mechanical simulation (baseline=48.7±0.9 g vs day 19=30.7±1.8 g), but not to thermal or cold stimuli. Therefore, only responses to mechanical stimulation were measured for the remainder of the study. L-29 at 10 mg kg−1 caused a significant (P<0.01) increase in paw withdrawal threshold at t=20–100 min post-injection as compared to vehicle and pre-injection values (Figure 3a).
Figure 3.
The effect of L-29 (10 mg kg−1) on hindpaw hypersensitivity to a mechanical stimulus in ddC- and VZV-treated rats. Hindpaw withdrawal thresholds in response to mechanical stimulation in (a) ddC-treated rats at day 19 post-initial treatment (n=12) and (b) VZV-treated rats at day 14 post-treatment (n=10) following L-29 (10 mg kg−1) or vehicle control. Statistical significance of differences between L-29 and vehicle control values (*P<0.01) was determined by a one-way ANOVA with Dunn's all pairwise multiple comparisons or (§P<0.01) between each post-injection value vs the pre-injection values (at time 0) using a one way ANOVA with Dunnett's multiple comparisons vs control post hoc analysis. Each value is the mean±s.e.m. ANOVA, analysis of variance; ddC, dideoxycitadine; VZV, varicella zoster virus.
L-29 (10 mg kg−1) significantly attenuates mechanical hypersensitivity in VZV-treated animals
By day 14 post-VZV injection, 50% of animals developed a significant (P<0.01) ipsilateral hindpaw withdrawal sensitivity to mechanical simulation (baseline=47.8±1.1 g vs day 14=27.0±1.1 g) and were therefore included in the study. In line with previous studies (Hasnie et al., 2007), no hypersensitivity developed to thermal or cold stimuli and therefore, once again only responses to mechanical stimulation were measured for the remainder of the study. L-29 at 10 mg kg−1 caused a significant (P<0.01) increase in paw withdrawal threshold at 40 and 60 min post-injection as compared to vehicle and at 20–80 min post-injection as compared to pre-injection values (Figure 3b).
L-29 is comparable to gabapentin in attenuating behaviour reflex hypersensitivity in rodent models of neuropathic pain
To assess the effect of L-29, as compared to a commonly employed analgesic, we compared the effect of L-29 at 10 mg kg−1 on hindpaw withdrawal thresholds in all three rodent models to that of gabapentin (50 mg kg−1). In all animals, there was no significant difference in values between the vehicle for L-29 (ethanol/cremophor) and the vehicle for gabapentin (saline), therefore the vehicle values are pooled.
In PSNI-treated animals, gabapentin significantly attenuated (P<0.01) thermal hypersensitivity at 20–100 min post-injection as compared to vehicle control and as compared to pre-injection values (Figure 4a). At all time points, there was no significant difference between the effect of L-29 and gabapentin. Gabapentin also significantly (P<0.01) attenuated mechanical hypersensitivity at 40–100 min post-injection as compared to vehicle control and as compared to pre-injection values (Figure 4b). At 40–100 min post-injection, there was no significant difference between gabapentin and L-29 values. At 20 min post-injection, L-29 withdrawal values were significantly (P<0.01) higher than that of gabapentin.
Figure 4.
The effect of gabapentin (50 mg kg−1) vs L-29 (10 mg kg−1) on hindpaw reflex behaviours in PSNI-, ddC- and VZV-treated rats. Hindpaw withdrawal thresholds to (a) thermal or (b) mechanical stimulus in PSNI rats treated (c) mechanical stimulus in ddC-treated rats and (d) mechanical stimulus in VZV-treated rats following injection with L-29 (10 mg kg−1) or gabapentin (50 mg kg−1) (n=12 per group). Statistical significance of differences (*P<0.01) of each drug vs vehicle control or (§P<0.05) between L-29 vs gabapentin was determined by a one-way ANOVA with Dunn's all pairwise multiple comparisons post hoc analysis. Each value is the mean±s.e.m. ANOVA, analysis of variance; ddC, dideoxycitadine; PSNI, partial sciatic nerve injury; VZV, varicella zoster virus.
In ddC-treated animals, gabapentin significantly (P<0.01) attenuated mechanical hypersensitivity at 20–100 min post-injection as compared to vehicle control and as compared to pre-injection values (Figure 4c). At 40–100 min post-injection, there was no significant difference between gabapentin and L-29 values. At 20 min post-injection, L-29 withdrawal values were significantly (P<0.05) higher than that of gabapentin.
In VZV-treated animals, gabapentin significantly (P<0.05) attenuated mechanical hypersensitivity at 100 min post-injection as compared to vehicle control and as compared to pre-injection values (Figure 4d). At 40 and 60 min post-injection, L-29 withdrawal values were significantly (P<0.01) higher than those of gabapentin.
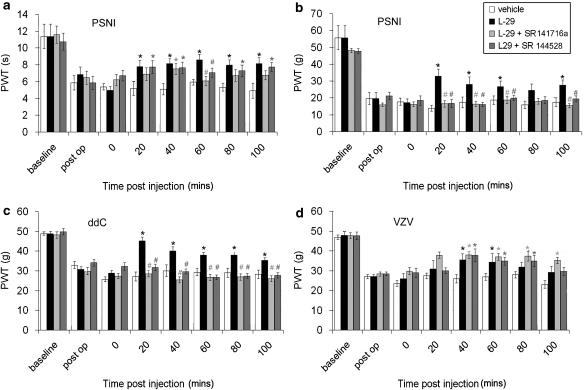
The cannabinoid receptor antagonists SR141716a and SR144528 had selective effects on hypersensitivity associated with rodent models of neuropathic pain
To investigate the possible site of action of L-29-mediated effects, we assessed the effects of the CB1 receptor antagonist SR141716a and the CB2 receptor antagonist SR144528 on L-29-mediated analgesia. When administered at 1 mg kg−1, 2 min before L-29, SR141716a completely abolished the effect of L-29 on thermal hypersensitivity in PSNI-treated animals only at 60 min post-injection (Figure 5a). At 20, 80 and 100 min post-injection, the effect of SR141716a+L-29 was not significantly different from either; L-29 alone or vehicle values, suggesting it is having a partial effect and therefore results in withdrawal thresholds between that of the vehicle or L-29. At 40 min post-injection, SR141716a+ L-29 was not significantly different from L-29 alone and was significantly (P<0.05) higher than vehicle values.
Figure 5.
The effect of the cannabinoid antagonists SR141716a and SR144528 on the response to L-29 in PSNI-, ddC- or VZV-treated rats. Hindpaw withdrawal thresholds to (a) thermal or (b) mechanical stimulus in PSNI rats; (c) mechanical stimulus in ddC-treated rats and (d) mechanical stimulus in VZV-treated rats, following injection with L-29 (10 mg kg−1) or SR141716A (1 mg kg−1)+L-29 (10 mg kg−1) or SR144528 (1 mg kg−1)+L-29 (10 mg kg−1) (n=10 per group). The statistical significance of difference (*P<0.05) between drug and vehicle control or (#P<0.05) the antagonist and L-29 was determined by a one-way ANOVA with Dunn's all pairwise multiple comparisons post hoc analysis. Each value is the mean±s.e.m. ANOVA, analysis of variance; ddC, dideoxycitadine; PSNI, partial sciatic nerve injury; VZV, varicella zoster virus.
The CB2 receptor antagonist SR144528, given at 1 mg kg−1, 2 min before L-29, had no effect on L-29-induced attenuation of thermal hypersensitivity in PSNI animals at 20, 40, 80 and 100 min post-injection (Figure 5a). At 60 min post-injection, the effect of SR144528+L-29 is not significantly different from either L-29 alone or vehicle treatment again suggesting a partial effect. In contrast, pre-treatment with either SR141716a or SR144528 completely abolished the effects of L-29 on mechanical hypersensitivity in PSNI animals over the entire time tested (Figure 5b). At all time points, SR141716a+L-29 or SR144528+L-29 values were not significantly different to vehicle values. Likewise, pre-treatment with either SR141716a or SR144528 completely abolished the effects of L-29 on mechanical hypersensitivity in ddC-treated animals over the entire time tested. At all time points, SR141716a+L-29 or SR144528+L-29 values were not significantly different to vehicle values (Figure 5c).
In VZV-treated rats, pre-treatment with SR141716a or SR144528 had no significant effect on withdrawal values with L-29 alone across the entire time tested (Figure 5d).
The PPAR-α receptor antagonist MK-886 significantly reduced the effect of L-29 on reflex withdrawal thresholds in the PSNI model of neuropathic pain
To further investigate the possible site of action of L-29-mediated effects, we assessed the effects of the PPAR-α receptor antagonist MK-886 (Kehrer et al., 2001; Verri et al., 2005; Collino et al., 2006) on L-29-mediated analgesia in the PSNI model. When administered at 1 mg kg−1, 2 min before L-29, MK-886 significantly (P<0.01) reversed the effect of L-29 on thermal hypersensitivity at 60 min post-injection. However, at 40 and 100 min post-injection, the effect of MK-866 was not significant from either L-29 alone or vehicle suggesting a partial effect (Figure 6a). MK-886 also significantly (P<0.01) reversed the effect of L-29 on mechanical hypersensitivity in PSNI-treated animals at 20, 40 and 100 min post-injection (Figure 6b). At 20 and 40 min post-injection, the effect of MK-866+L-29 was comparable to vehicle, whereas at 100 min, it was significantly less than L-29 alone, but significantly greater than vehicle suggesting a partial attenuation. Likewise, at 60 min MK-866+L-29 was not significantly different from either L-29 or vehicle alone indicating partial effects on the action of L-29.
Figure 6.
The effect of the PPAR-α antagonist MK-886 on the response to L-29 in PSNI-treated rats. Hindpaw withdrawal thresholds to (a) thermal or (b) mechanical stimulus in PSNI rats treated following injection with L-29 (10 mg kg−1) or MK-886 (1 mg kg−1)+L-29 (10 mg kg−1) (n=10 per group). The statistical significance of difference (*P<0.01) between drug and vehicle control or (#P<0.01) L-29+MK-886 and L-29 was determined by a one-way ANOVA with Dunn's all pairwise multiple comparisons post hoc analysis. Each value is the mean±s.e.m. ANOVA, analysis of variance; PSNI, partial sciatic nerve injury; PPAR, peroxisome proliferator-activated receptor-α.
L-29 effectively reversed thigmotactic behaviour displayed by PSNI animals in the open field arena
As a novel measure of pain-related behaviour that does not rely on reflex thresholds, we have employed the open field paradigm, which has been used extensively for the assessment of anxiolytic agents in rodents (Carli et al., 1989; Holmes, 2001; Cryan and Holmes, 2005). Previous characterisation of rodent models of neuropathic pain have demonstrated that animals with significant hypersensitivity to reflex withdrawal tests demonstrated significant thigmotactic (‘wall hugging') behaviour reflected by a significant decrease in both number of entries into and time spent in the inner zone that is responsive to classically employed analgesics (Wallace et al., 2006; Hasnie et al., 2007). Therefore, assessment of novel drug compounds using this paradigm can give further insight into their potential as analgesics in humans. At day 7 post-surgery, PSNI rats that displayed significant behavioural reflex sensitivity changes to mechanical, thermal and cold stimuli were treated with vehicle control (ethanol/cremophor) 20 min before exposure to the open field. These rats displayed significant thigmotactic (‘wall hugging') behaviour of a magnitude similar to other neuropathic pain models reflected by a significant (P<0.01) decrease in both number of entries and time spent in the inner zone as compared to day 7 sham control animals (Figures 7a and b). Pre-treatment with L-29 (10 mg kg−1) caused a significant (P<0.05) increase in both the number of entries and the time spent in the inner zone, such that they were not significantly different from sham animals. Furthermore, the total distance travelled was not significantly different between all groups (Figure 7c), indicating a lack of motor or cognitive impairment in the altered behaviour.
Figure 7.
The effect of L-29 (10 mg kg−1) on altered spontaneous exploratory activity in the open field arena in rats 7 days following PSNI. (a) The number of entries into the inner zone (40 × 40 cm) and (b) time spent in the inner zone of the open field arena was significantly reduced in rats 7 days following PSNI treatment as compared to sham (n=10 per group). This reduction was reversed in animals treated with L-29 (10 mg kg−1) 20 min before the test as compared to vehicle-treated rats. (c) The total distance moved within the open field arena (1 × 1 m) was assessed over 15 min and is not significantly different between sham control animals, vehicle-treated PSNI animals or L-29-treated PSNI animals. The statistical significance of differences from the vehicle control group (*P<0.05) was determined using a one way ANOVA with Dunn's all pairwise multiple comparisons. Each value is the mean±s.e.m. (d, e) Example traces of (d) vehicle-treated and (e) L-29-treated PSNI rats over 15 min in the open field arena. ANOVA, analysis of variance; PSNI, partial sciatic nerve injury.
Discussion
In the present study, we have demonstrated that acute systemic administration of the PEA analogue, L-29, reduces hypersensitivity associated with nerve injury, antiretroviral treatment or injection of VZV in to the hindpaw. We have demonstrated a potential role for CB receptors and the PPAR-α receptor in L-29 effects. Additionally, nerve injury-related thigmotactic behaviour was inhibited by L-29 without overt effects on motor performance. This suggests that L-29 was analgesic in a variety of pain states, without producing the side effects generally associated with CB receptor agonist administration.
Anandamide, 2-AG and endogenous fatty acid amides, such as PEA, have been shown to have analgesic properties in several different animal models of pain. The major mechanism terminating the actions of anandamide and one of those terminating the actions of PEA is hydrolysis by the enzyme, FAAH. L-29, synthesised via modification of the ethyl head chain in N-palmitoylethanolamine, inhibits the metabolism of anandamide in vitro (Vandevoorde et al., 2003a), most likely via inhibition of FAAH activity. Other FAAH inhibitors have been shown to potentiate the pharmacological actions of anandamide in vitro and in vivo (Childers et al., 1994; Pertwee et al., 1995) as well as to elevate levels of 2-AG (de Lago et al., 2005; Jhaveri et al., 2006; Maione et al., 2006). The proposal that FAAH inhibition enhances the analgesic effects of these endocannabinoids is supported by the finding that FAAH knockout mice (Cravatt et al., 2001) display a hypoalgesic phenotype and by the production of analgesia in rodent pain models by the FAAH inhibiting compounds; OL135 and URB597 (Lichtman et al., 2004a; Chang et al., 2006; Jayamanne et al., 2006; Jhaveri et al., 2006).
We have therefore assessed the analgesic ability of L-29 in a variety of rodent models of neuropathic pain including, a model of nerve injury-induced neuropathic pain, the PSNI model (Seltzer et al., 1990), a model of antiretroviral (ddC)-associated neuropathic pain (Joseph et al., 2004; Wallace et al., 2006) and a model of varicella zoster-associated pain (Garry et al., 2005; Hasnie et al., 2007), which correlates with mechanisms of post-herpetic neuralgia (PHN) in humans. These three models have very distinct initiating insults, but share the common behavioural outcome of hypersensitivity to a punctate mechanical stimulus and thigmotaxis. In such models, we hope to highlight the therapeutic potential of L-29 as an analgesic in a number of aetiologies associated with neuropathic pain.
L-29 reversed signs of punctate mechanical hypersensitivity in all models of neuropathic pain and thermal hypersensitivity in the PSNI model. Importantly, L-29 was as effective, and in the case of VZV animals, more effective than gabapentin (50 mg kg−1), an analgesic which has been shown to be effective in rodent models of neuropathy (Abdi et al., 1998; De Vry et al., 2004; Donovan-Rodriguez et al., 2005) and in a number of human neuropathic pain conditions, including HIV-neuropathy and PHN (Finnerup et al., 2005; Hempenstall et al., 2005). This highlights the potential clinical efficacy of L-29.
The exact mechanisms of L-29-induced analgesia remains to be determined. The inhibition of the metabolism of anandamide in vitro (Vandevoorde et al., 2003a) suggested that L-29 would act as a FAAH inhibitor. PEA is also degraded by a second enzyme, NAAA (Tsuboi et al., 2005) and therefore it remains a possibility that L-29 can act to inhibit the action of this enzyme also with the similar effect of increasing endogenous PEA. However, further studies are required to fully assess the effect of L-29 levels of FAAH activity, NAAA activity and the ensuing effect on levels of endogenous cannabinoids in vivo.
A potential mechanism of action addressed by this study is the activation of CB receptors. Anandamide is a weak partial agonist at CB1 receptors, which are expressed throughout the peripheral and central sensory nervous system. There is now a large body of evidence supporting CB1-mediated antinociceptive and antihyperalgesic effects (Richardson et al., 1998; Bridges et al., 2001; Farquhar-Smith and Rice, 2001; Wallace et al., 2003). Evidence supports a direct involvement of the CB1 receptor in the analgesic phenotype of FAAH knockout mice (Cravatt et al., 2001) and in OL135-mediated FAAH inhibition in the formalin test (Lichtman et al., 2004b). In contrast, reversal of mechanical hypersensitivity in a model of mild thermal injury or sciatic nerve ligation did not appear to have a CB1 receptor-mediated component (Chang et al., 2006). The CB2 receptor also mediates the analgesic effects of endocannabinoids (Malan et al., 2001, 2003) and has been shown to play a role in inflammatory and neuropathic pain states (Farquhar-Smith and Rice, 2001; Farquhar-Smith et al., 2002; Ibrahim et al., 2003; Scott et al., 2004) as well as analgesia in FAAH−/− mice (Lichtman et al., 2004a). A further CB receptor may exist because, although PEA does not have high affinity for CB2 receptors, the analgesic actions of PEA are consistently antagonised by the CB2 receptor antagonist SR144528 (Calignano et al., 1998, 2001; Farquhar-Smith et al., 2002). PEA may therefore act via hitherto uncharacterised CB2-like receptors, at which SR144528 is also a functional antagonist (LoVerme et al., 2005b).
Therefore, we assessed the role of CB receptors in L-29-induced analgesia using selective CB1 and CB2 receptor antagonists. The effect of the antagonists varied depending on model type and modality tested, suggesting that different mechanisms downstream of FAAH are involved in these models and their manifestations of hypersensitivity. The greatest effect was on L-29 associated reversal of hypersensitivity to punctate mechanical stimulation in PSNI and ddC-treated animals, which was abolished by both antagonists. Although anandamide, PEA and L-29 do not have high affinity for the CB2 receptor, there are a number of explanations as to why SR144528 produced an effect. First, although a potent CB2 antagonist, SR144528 has also been associated with CB1 receptor antagonism at relatively low doses (Griffin et al., 1999; Iwamura et al., 2001). As we cannot quantify the concentration of SR144528 in our in vivo experiments, it is possible that it is acting at the CB1 receptor. This may explain why in both the PSNI and ddC models, both antagonists have equal effect when used alone. Second, as mentioned above, SR144528 may be antagonising a hitherto unrecognised ‘CB2-like' receptor, which is activated by PEA and perhaps also directly by L-29. Nevertheless, these data suggest that the endocannabinoid system is directly involved in L-29-induced analgesia and may provide a useful therapeutic target. In contrast, CB receptor blockade had no consequences for the L-29-associated analgesia in VZV-treated animals. VZV-associated hypersensitivity is thus likely to involve a different pathophysiology from that of nerve injury or drug-induced neuropathic models, which may account for mechanistic differences in response to L-29.
These results suggest that L-29 or the endocannainoids affected by L-29 are acting, at least in part, via CB receptor-independent mechanisms. This may include L-29 acting directly on alternative receptors or via the promotion of known endocannabinoid-CB receptor-independent mechanisms. L-29 is a structural analogue of PEA and therefore may act mechanistically in a manner similar to PEA. The molecular target for PEA is controversial and evidence would suggest that it is not a ligand at either known CB receptors (LoVerme et al., 2005b). However, it is thought that PEA mediates its anti-inflammatory and some of its analgesic actions through activation of PPAR-α (LoVerme et al., 2005a, 2006). In line with this, the analgesic effect of L-29 was diminished by blockade of the PPAR-α receptor, suggesting that L-29 may be acting directly via this receptor or indirectly by increasing endogenous levels of PEA.
The possible involvement of CB receptor-independent mechanisms are supported by the fact that anandamide is thought to be a ligand at various targets relevant to transmission and modulation of nociceptive information including; transient receptor potential vanilloid type-1 receptors (TRPV-1), 5-HT receptors, voltage-gated calcium channels, and the NMDA receptor (Fan, 1995; Mackie et al., 1995; Twitchell et al., 1997; Hampson et al., 1998; Barann et al., 2002; Oz et al., 2002; Roberts et al., 2002). L-29 is known not to directly activate TRPV1 (Vandevoorde et al., 2003b). However, it is possible that anandamide, the levels of which may be affected by L-29, may be acting upon these receptors and could therefore potentially become pro-nociceptive, in addition to being analgesic. We found the analgesic effect of L-29 at the highest does tested (20 mg kg−1) to be less than at the optimum dose of 10 mg kg−1. This could represent that at higher doses, L-29 causes anandamide accumulation of a magnitude great enough to activate pro-nociceptive targets such as TRPV-1 thereby counteracting analgesic effects via CB receptors. Therefore, further in-depth investigation of these receptor types would be of interest.
Finally, L-29 affected measures of spontaneous exploratory activity and thigmotaxis in the open field paradigm, which is classically used as a measure of anxiety related behaviour (Carli et al., 1989; Holmes, 2001; Cryan and Holmes, 2005). Such altered patterns of behaviour are associated with rodent models of pain (Wallace et al., 2007) and are sensitive to clinically employed analgesics, such as gabapentin and morphine (Wallace et al., 2006; Hasnie et al., 2007), suggesting that it represents a measure of non-stimulus evoked pain-like behaviour, either spontaneous pain or pain co-morbidities. Such measures are likely to prove more clinically relevant due to the fact that the majority of neuropathic pain patients present primarily with spontaneous pain with a lesser degree of hyperalgesia or allodynia (for a review, see (Mogil and Crager, 2004)). Moreover, pain co-morbidities such as anxiety and depression have a huge impact on neuropathic pain (Dworkin and Gitlin, 1991; Meyer-Rosberg et al., 2001) and therefore merit investigation. The reversal of thigmotactic behaviour in PSNI-treated animals by L-29 suggests that, in addition to modulating simple reflex pain pathways, it modulates higher levels of pain processing. Importantly, locomotor activity was unaffected by L-29, implying a lack of adverse central side effects that are often associated with CB receptor activation (Jarbe et al., 2004).
In conclusion, these data highlight the potential of PEA analogues as analgesics that are associated with fewer unacceptable side effects than globally acting cannabinoid agents.
Acknowledgments
These studies were supported by IC Innovations (London), the Charcot Foundation (Belgium) and a FRSM grant from the Belgian National Fund for Scientific Research.
Abbreviations
- CB
cannabinoid receptor
- cpe
cytopathic effect
- ddC
dideoxycitadine
- FAAH
fatty acid amide hydrolase
- Hel
human embryonic lung
- HIV
human immunodeficiency virus
- L-29
palmitoylallylamide
- MTI
mild thermal injury
- NAAA
N-acetylethanolamine acid hydrolase
- PEA
palmitoylethanolamide
- PPAR-α
peroxisome proliferator-activated receptor-α
- PHN
post-herpetic neuralgia
- PSNI
partial sciatic nerve injury
- SNL
sciatic nerve ligation
- THC
Δ9-tetrahydrocannabinol
- TRPV-1
transient receptor potential vanilloid type-1 receptors
- 2-AG
2-arachidonoyl glycerol
- VZV
varicella zoster virus
Conflict of interest
Professor Lambert and Dr Rice and their respective Universities hold a patent with respect to L-29.
References
- Abdi S, Lee DH, Chung JM. The anti-allodynic effects of amitriptyline, gabapentin, and lidocaine in a rat model of neuropathic pain. Anesth Analg. 1998;87:1360–1366. [PubMed] [Google Scholar]
- Ahmad KS, Rice ASC.Von Frey hairs compared with a force transducer for sensory measurement in normal and neuropathic rats 1999Poster Presentation at The Society for Neuroscience Annual Meeting Miami, FL
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, et al. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J. 2001;15:300–302. doi: 10.1096/fj.00-0399fje. [DOI] [PubMed] [Google Scholar]
- Barann M, Moldering SG, Bruss M, Bonisch H, Urban BW, Gothert M. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br J Pharmacol. 2002;137:589–596. doi: 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. 1998;353:23–31. doi: 10.1016/s0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- Bridges D, Ahmad K, Rice ASC. The synthetic cannabinoid WIN55, 212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br J Pharmacol. 2001;133:586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calignano A, La RanA G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Piomelli D. Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide. Eur J Pharmacol. 2001;419:191–198. doi: 10.1016/s0014-2999(01)00988-8. [DOI] [PubMed] [Google Scholar]
- Carli M, Prontera C, Samanin R. Effect of 5-HT1A agonists on stress-induced deficit in open field locomotor activity of rats: evidence that this model identifies anxiolytic-like activity. Neuropharmacology. 1989;28:471–476. doi: 10.1016/0028-3908(89)90081-6. [DOI] [PubMed] [Google Scholar]
- Chang L, Luo L, Palmer JA, Sutton S, Wilson SJ, Barbier AJ, et al. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. Br J Pharmacol. 2006;148:102–113. doi: 10.1038/sj.bjp.0706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR, Sexton T, Roy MB. Effects of anandamide on cannabinoid receptors in rat brain membranes. Biochem Pharmacol. 1994;47:711–715. doi: 10.1016/0006-2952(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Collino M, Aragno M, Mastrocola R, Benetti E, Gallicchio M, Dianzani C, et al. Oxidative stress and inflammatory response evoked by transient cerebral ischemia/reperfusion: effects of the PPAR-alpha agonist WY14643. Free Radic Biol Med. 2006;41:579–589. doi: 10.1016/j.freeradbiomed.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Conti S, Costa B, Colleoni M, Parolaro D, Giagnoni G. Antiinflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br J Pharmacol. 2002;135:181–187. doi: 10.1038/sj.bjp.0704466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Dalziel RG, Bingham S, Sutton D, Grant D, Champion JM, Dennis SA, et al. Allodynia in rats infected with varicella zoster virus – a small animal model for post-herpetic neuralgia. Brain Res Brain Res Rev. 2004;46:234–242. doi: 10.1016/j.brainresrev.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Izzo AA, Degenhardt B, Valenti M, Scaglione G, Capasso R, et al. Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: review of the available pre-clinical data, and first human studies. Neuropharmacology. 2005;48:1154–1163. doi: 10.1016/j.neuropharm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- De Lago E, Petrosino S, Valenti M, Morera E, Ortega-Gutierrez S, Fernandez-Ruiz J, et al. Effect of repeated systemic administration of selective inhibitors of endocannabinoid inactivation on rat brain endocannabinoid levels. Biochem Pharmacol. 2005;70:446–452. doi: 10.1016/j.bcp.2005.05.011. [DOI] [PubMed] [Google Scholar]
- De Vry J, Kuhl E, Franken-Kunkel P, Eckel G. Pharmacological characterization of the chronic constriction injury model of neuropathic pain. Eur J Pharmacol. 2004;491:137–148. doi: 10.1016/j.ejphar.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Donovan-Rodriguez T, Dickenson AH, Urch CE. Gabapentin normalizes spinal neuronal responses that correlate with behavior in a rat model of cancer-induced bone pain. Anesthesiology. 2005;102:132–140. doi: 10.1097/00000542-200501000-00022. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Gitlin MJ. Clinical aspects of depression in chronic pain patients. Clin J Pain. 1991;7:79–94. doi: 10.1097/00002508-199106000-00004. [DOI] [PubMed] [Google Scholar]
- Fan P. Cannabinoid agonists inhibit the activation of 5-HT3 receptors in rat nodose ganglion neurons. J Neurophysiol. 1995;73:907–910. doi: 10.1152/jn.1995.73.2.907. [DOI] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Jaggar SI, Rice ASC. Attenuation of nerve growth factor-induced visceral hyperalgesia via cannabinoid CB1 and CB2-like receptors. Pain. 2002;97:11–21. doi: 10.1016/s0304-3959(01)00419-5. [DOI] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Rice ASC. Administration of endocannabinoids prevents a referred hyperalgesia associated with inflammation of the urinary bladder. Anesthesiology. 2001;94:507–513. doi: 10.1097/00000542-200103000-00023. [DOI] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Rice ASC. A novel neuroimmune mechanism in cannabinoid-mediated attenuation of nerve growth factor-induced hyperalgesia. Anesthesiology. 2003;99:1391–1401. doi: 10.1097/00000542-200312000-00024. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Otto M, McQua YHJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118:289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker SM, Quinn JP, Wallace C, Blackburn-Munro G, Kelly BG, Fiskerstrand CE, et al. Behavioural changes in the rat following infection with varicella-zoster virus. J Genet Virol. 1999;80:2433–2436. doi: 10.1099/0022-1317-80-9-2433. [DOI] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, et al. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Garry EM, Delaney A, Anderson HA, Sirinathsinghji EC, Clapp RH, Martin WJ, et al. Varicella zoster virus induces neuropathic changes in rat dorsal root ganglia and behavioral reflex sensitisation that is attenuated by gabapentin or sodium channel blocking drugs. Pain. 2005;118:97–111. doi: 10.1016/j.pain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Giang DK, Cravat TBF. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci USA. 1997;94:2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G, Wray EJ, Tao Q, McAllister SD, Rorrer WK, Aung MM, et al. Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: further evidence for cannabinoid CB2 receptor absence in the rat central nervous system. Eur J Pharmacol. 1999;377:117–125. doi: 10.1016/s0014-2999(99)00402-1. [DOI] [PubMed] [Google Scholar]
- Hampson AJ, Bornheim LM, Scanziani M, Yost CS, GraY AT, Hansen BM, et al. Dual effects of anandamide on NMDA receptor-mediated responses and neurotransmission. J Neurochem. 1998;70:671–676. doi: 10.1046/j.1471-4159.1998.70020671.x. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown FFC, Jovis J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, et al. Further characterization of a rat model of varicella zoster virus-associated pain: Relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience. 2007;144:1495–1508. doi: 10.1016/j.neuroscience.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyes Z, Nemeth J, Than M, Bolcskei K, Pinter E, SzolcsanyI J. Inhibitory effect of anandamide on resiniferatoxin-induced sensory neuropeptide release in vivo and neuropathic hyperalgesia in the rat. Life Sci. 2003;73:2345–2353. doi: 10.1016/s0024-3205(03)00651-9. [DOI] [PubMed] [Google Scholar]
- Hempenstall K, Nurmikko TJ, Johnson RW, A'hern RP, Rice ASC. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2:e164. doi: 10.1371/journal.pmed.0020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg U, Eliav E, Bennett GJ, Kopin IJ. The analgesic effects of R(+)-Win 55212'2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. pp. Neurosci Letts. 1997;221:157–160. doi: 10.1016/s0304-3940(96)13308-5. [DOI] [PubMed] [Google Scholar]
- Hohmann AG. Inhibitors of monoacylglycerol lipase as novel analgesics. Br J Pharmacol. 2007;150:673–675. doi: 10.1038/sj.bjp.0707153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann A, Suplita R. Endocannabinoid mechanisms of pain modulation. AAPS J. 2006;8:E693–E708. doi: 10.1208/aapsj080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A. Targeted gene mutation approaches to the study of anxiety-like behavior in mice. Neurosci Biobehav Rev. 2001;25:261–273. doi: 10.1016/s0149-7634(01)00012-4. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura H, Suzuki H, Ueda Y, Kaya T, Inaba T. In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J Pharmacol Exp Ther. 2001;296:420–425. [PubMed] [Google Scholar]
- Jaggar SI, Hasnie FS, Sellaturay S, Rice ASC. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Dipatrizio NV, Lu D, Makriyannis A. Adamantyl-delta8-tetrahydrocannabinol (AM-411), a selective cannabinoid CB1 receptor agonist: effects on open-field behaviors and antagonism by SR-141716 in rats. Behav Pharmacol. 2004;15:517–521. doi: 10.1097/00008877-200411000-00008. [DOI] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, RichardsoN D, Kendall DA, Barrett DA, Chapman V. Analgesic effects of fatty acid amide hydrolase inhibition in a rat model of neuropathic pain. J Neurosci. 2006;26:13318–13327. doi: 10.1523/JNEUROSCI.3326-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Chen X, Khasar SG, Levine JD. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain. 2004;107:147–158. doi: 10.1016/j.pain.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Kehl LJ, Hamamoto DT, Wacnik PW, Croft DL, Norsted BD, Wilcox GL, et al. A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain. 2003;103:175–186. doi: 10.1016/s0304-3959(02)00450-5. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Biswal SS, La E, Thuillier P, Datta K, Fischer SM, et al. Inhibition of peroxisome-proliferator-activated receptor (PPAR)alpha by MK886. Biochem J. 2001;356:899–906. doi: 10.1042/0264-6021:3560899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DM, Di Marzo V. The palmitoylethanolamide and oleamide enigmas: are these two fatty acid amides cannabimimetic. Curr Med Chem. 1999;6:757–773. [PubMed] [Google Scholar]
- Lambert DM, Vandevoorde S, Jonsson KO, Fowler CJ. The palmitoylethanolamide family: a new class of anti-inflammatory agents. Curr Med Chem. 2002;9:663–674. doi: 10.2174/0929867023370707. [DOI] [PubMed] [Google Scholar]
- Leung D, Du W, Hardouin C, Cheng H, Hwang I, Cravatt BF, et al. Discovery of an exceptionally potent and selective class of fatty acid amide hydrolase inhibitors enlisting proteome-wide selectivity screening: concurrent optimization of enzyme inhibitor potency and selectivity. Bioorg Med Chem Lett. 2005;15:1423–1428. doi: 10.1016/j.bmcl.2004.12.085. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, et al. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther. 2004a;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004b;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005a;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- LoVerme J, LA Rana G, Russo R, Calignano A, Piomelli D. The search for the palmitoylethanolamide receptor. Life Sci. 2005b;77:1685–1698. doi: 10.1016/j.lfs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006;319:1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- MacKie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione S, Bisogno T, De NV, Palazz OE, Cristino L, Valenti M, et al. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther. 2006;316:969–982. doi: 10.1124/jpet.105.093286. [DOI] [PubMed] [Google Scholar]
- Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, et al. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Malan TP, Jr, Ibrahim MM, Lai J, Vanderah TW, Makriyannis A, Porreca F. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects. Curr Opin Pharmacol. 2003;3:62–67. doi: 10.1016/s1471-4892(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Meyer-Rosberg K, Kvarnstrom A, Kinnman E, Gordh T, Nordfors LO, Kristofferson A. Peripheral neuropathic pain – a multidimensional burden for patients. Eur J Pain. 2001;5:379–389. doi: 10.1053/eujp.2001.0259. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals. Pain. 2004;112:12–15. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Moller KA, Johansson B, Berge OG. Assessing mechanical allodynia in the rat paw with a new electronic algometer. J Neurosci Methods. 1998;84:41–47. doi: 10.1016/s0165-0270(98)00083-1. [DOI] [PubMed] [Google Scholar]
- Oz M, Zhang L, Morales M. Endogenous cannabinoid, anandamide, acts as a noncompetitive inhibitor on 5-HT3 receptor-mediated responses in Xenopus oocytes. Synapse. 2002;46:150–156. doi: 10.1002/syn.10121. [DOI] [PubMed] [Google Scholar]
- Patel S, Carrier EJ, Ho WS, Rademacher DJ, Cunningham S, Reddy DS, et al. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46:342–349. doi: 10.1194/jlr.M400377-JLR200. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Fernando SR, Griffin G, Abadji V, Makriyannis A. Effect of phenylmethylsulphonyl fluoride on the potency of anandamide as an inhibitor of electrically evoked contractions in two isolated tissue preparations. Eur J Pharmacol. 1995;272:73–78. doi: 10.1016/0014-2999(94)00618-h. [DOI] [PubMed] [Google Scholar]
- Petrosino S, Palazzo E, De NV, Bisogno T, Rossi F, Maione S, et al. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology. 2007;52:415–422. doi: 10.1016/j.neuropharm.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Rice AS. Cannabinoids and pain. Curr Opin Investig Drugs. 2001;2:399–414. [PubMed] [Google Scholar]
- Rice AS, Farquhar-Smith WP, Nagy I. Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot Essent Fatty Acids. 2002;66:243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- Rice ASC. Melzack and Wall: Textbook of Pain. Elsevier: London; 2005. Cannabinoids; pp. 521–540. [Google Scholar]
- Rice ASC, Lever I, Zarnegar R. Cannabinoids and Analgesia, with Special Reference to Neuropathic Pain 2007IASP Press: Seattle; In: McQuay HJ and Moore RA (eds)(in press) [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Christie MJ, Connor M. Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons. Br J Pharmacol. 2002;137:421–428. doi: 10.1038/sj.bjp.0704904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, Wright CE, Angus JA. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109:124–131. doi: 10.1016/j.pain.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Smith FL, Fujimori K, Lowe J, Welch SP. Characterization of delta9-tetrahydrocannabinol and anandamide antinociception in nonarthritic and arthritic rats. Pharmacol Biochem Behav. 1998;60:183–191. doi: 10.1016/s0091-3057(97)00583-2. [DOI] [PubMed] [Google Scholar]
- Smith GD, Harrison SM, Birch PJ, Elliot PJ, Malcangio M, Bowery NG. Increased sensitivity to the antinociceptive activity of (+/−)- baclofen in an animal model of chronic neuropathic, but not chronic inflammatory hyperalgesia. Neuropharmacology. 1994;33:1103–1108. doi: 10.1016/0028-3908(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Ueda N. Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J Biol Chem. 2005;280:11082–11092. doi: 10.1074/jbc.M413473200. [DOI] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- Vandevoorde S, Jonsson KO, Fowler CJ, Lambert DM. Modifications of the ethanolamine head in N-palmitoylethanolamine: synthesis and evaluation of new agents interfering with the metabolism of anandamide. J Med Chem. 2003a;46:1440–1448. doi: 10.1021/jm0209679. [DOI] [PubMed] [Google Scholar]
- Vandevoorde S, Lambert DM, Smart D, Jonsson KO, Fowler CJ. N-Morpholino- and N-diethyl-analogues of palmitoylethanolamide increase the sensitivity of transfected human vanilloid receptors to activation by anandamide without affecting fatty acid amidohydrolase activity. Bioorg Med Chem. 2003b;11:817–825. doi: 10.1016/s0968-0896(02)00567-9. [DOI] [PubMed] [Google Scholar]
- Verri WA, Jr, Molina RO, Schivo IR, Cunha TM, Parada CA, Poole S, et al. Nociceptive effect of subcutaneously injected interleukin-12 is mediated by endothelin (ET) acting on ETB receptors in rats. J Pharmacol Exp Ther. 2005;315:609–615. doi: 10.1124/jpet.105.089409. [DOI] [PubMed] [Google Scholar]
- Wallace VC, Blackbeard J, Segerdahl A, Hasnie FS, Pheby T, McMahon SB, et al. Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy Pain 2007(In press) [DOI] [PMC free article] [PubMed]
- Wallace VC, Cottrell DF, Brophy PJ, Fleetwood-Walker SM. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J Neurosci. 2003;23:3221–3233. doi: 10.1523/JNEUROSCI.23-08-03221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VC, McMahon SB, Rice ASC.The Characterisation of a Rodent Model of Antiretroviral-Associated Painful Peripheral Neuropathy 2006Poster presentation at The Society for Neuroscience Annual Meeting, Atlanta, GA
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]