Abstract
Background and purpose:
α1-Adrenoceptor antagonists are extensively used in the treatment of hypertension and lower urinary tract symptoms associated with benign prostatic hyperplasia. Among the side effects, ejaculatory dysfunction occurs more frequently with drugs that are relatively selective for α1A-adrenoceptors compared with other drugs of this class. This suggests that α1A-adrenoceptors may contribute to ejaculation. However, this has not been studied at the molecular level.
Experimental approach:
The physiological contribution of each α1-adrenoceptor subtype was characterized using α1-adrenoceptor subtype-selective knockout (KO) mice (α1A-, α1B- and α1D-AR KO mice) since the subtype-specific drugs available are only moderately selective. We analysed the role of α1-adrenoceptors in the blood pressure and vascular response as well as ejaculation by determining these variables in α1-adrenoceptor subtype-selective KO mice and in mice with all their α1-adrenoceptor subtypes deleted (α1-AR triple-KO mice).
Key results:
The pregnancy rate was reduced by 50% in α1A-adrenoceptor KO mice, and this reduction was dramatically enhanced in α1-adrenoceptor triple-KO mice. Contractile tension of the vas deferens in response to noradrenaline was markedly decreased in α1A-adrenoceptor KO mice, and this contraction was completely abolished in α1-adrenoceptor triple-KO mice. This attenuation of contractility was also observed in the electrically stimulated vas deferens.
Conclusions and implications:
These results demonstrate that α1-adrenoceptors, particularly α1A-adrenoceptors, are required for normal contractility of the vas deferens and consequent sperm ejaculation as well as having a function in fertility.
Keywords: α1-Adrenoceptor, ejaculation, vas deferens
Introduction
α1-Adrenoceptors are stimulated by catecholamines released from sympathetic nerves and are known to have an important role in regulating the various physiological functions of the peripheral tissues. α1-Adrenoceptors have been classified into three subtypes, α1A, α1B and α1D, by molecular cloning and pharmacological analysis (McGrath, 1982; Han et al., 1987a; Foglar et al., 1995; Guarino et al., 1996). The contribution of each α1-adrenoceptor subtype to catecholamine-induced physiological responses was characterized in our previous studies using mice with targeted disruption of each subtype gene (α1A-, α1B- and α1D-adrenoceptor knockout (KO) mice), as the subtype-specific drugs available are only moderately selective and may interact with other adrenoceptors and non-adrenoceptors (Cavalli et al., 1997; Spreng et al., 2001; Drouin et al., 2002; Rokosh and Simpson, 2002; Tanoue et al., 2002a; O'Connell et al., 2003, 2006). Previous studies have clearly indicated that α1-adrenoceptors play an important role in the regulation of blood pressure (BP) (Cavalli et al., 1997; Rokosh and Simpson, 2002; Tanoue et al., 2002a), focal vascular drug responsiveness (Hosoda et al., 2005b), cardiac growth (O'Connell et al., 2003, 2006) and glucose homeostasis (Burcelin et al., 2004).
Since catecholamines cause vascular smooth muscle contraction by activating α1-adrenoceptors, α1-adrenoceptor antagonists were originally introduced for the treatment of hypertension. However, after the publication of several clinical trials, such as the ALLHAT (antihypertensive and lipid-lowering treatment to prevent heart attack; ALLHAT Collaborative Research Group, 2000) and V-HeFT (Cohn, 1988), these antagonists are no longer considered suitable for the primary treatment of this condition (Chobanian et al., 2003). Instead, α1-adrenoceptor antagonists are extensively used in the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (BPH) (van Dijk et al., 2006). While these antagonists are relatively safe drugs, some clinical studies have shown that ejaculatory dysfunction occurs during treatment with them (Debruyne, 2000; van Dijk et al., 2006). Since this side effect occurs more frequently with tamuslosin and silodosin, which are relatively selective for the α1A-subtype compared to other drugs of this class, α1A-adrenoceptors may contribute to ejaculatory function (Kawabe et al., 2006; van Dijk et al., 2006). A recent clinical study showed that acute treatment with tamuslosin reduces the mean ejaculatory volume by approximately 45%, and no sperm were detected in the midstream urine obtained after ejaculation (Hisasue et al., 2006; Hellstrom and Sikka, 2006). These studies indicate that α1-adrenoceptor antagonist-associated abnormal ejaculation may represent anejaculation rather than retrograde ejaculation. However, this has not been studied at the molecular level. Hence, in the present study, we analysed the role of α1-adrenoceptors in BP and vascular responses, as well as ejaculation, by use of mice with targeted disruption of each α1-adrenoceptor subtype as well as those with all α1-adrenoceptor subtypes deleted (α1-AR triple-KO mice). Our results demonstrated that α1-adrenoceptors, particularly α1A-adrenoceptors, are required for normal contractility in the vas deferens and consequent sperm ejaculation and hence have a function in fertility.
Methods
Gene-targeted mice
Each subtype-selective α1-adrenoceptor KO mouse (α1A-AR KO, α1B-AR KO and α1D-AR KO mice) was generated, backcrossed with a C57BL/6J mouse more than six times, and maintained on a C57BL/6J background (Cavalli et al., 1997; Rokosh and Simpson, 2002; Tanoue et al., 2002b). In order to generate mice with all α1-adrenoceptor subtypes deleted (α1-AR triple-KO), each subtype-selective α1-adrenoceptor KO mouse was crossbred. Since male α1-adrenoceptor triple-KO mice had a seriously defective breeding ability, the male mice that were heterozygous for the α1A-adrenoceptor gene and homozygous for both the α1B- and α1D-adrenoceptor genes (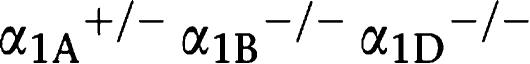 -AR KO mice;
-AR KO mice;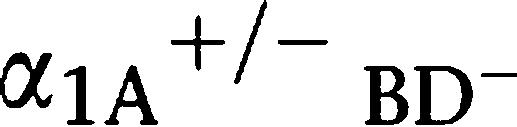 AR KO mice) were mated with female α1-adrenoceptor triple-KO mice. All mice were housed in micro-isolator cages in a pathogen-free barrier facility and placed on a 12 h light/dark cycle with access to food and water ad libitum, except when an experimental protocol was being followed. All data presented here were obtained from male mice. All experimental procedures followed the approved guidelines of this institute.
AR KO mice) were mated with female α1-adrenoceptor triple-KO mice. All mice were housed in micro-isolator cages in a pathogen-free barrier facility and placed on a 12 h light/dark cycle with access to food and water ad libitum, except when an experimental protocol was being followed. All data presented here were obtained from male mice. All experimental procedures followed the approved guidelines of this institute.
Genotyping was performed by PCR using genomic DNA from tail tissue, as described previously (Tanoue et al., 2002a). The expression level of the α1-adrenoceptors was examined by RT-PCR using a total RNA sample extracted from the aorta and sexual organs, such as the vas deferens, epidydimus, testis and seminal vesicle, as described previously (Tanoue et al., 2002b). The body weight, tissue weight and histological analysis were determined at 8–15 weeks of age, as described previously (Tanoue et al., 2002b).
Receptor binding study
A receptor binding study was performed as described previously (Hosoda et al., 2005a). In brief, whole brain was dissected from mice, placed in lysis buffer A (250 mM sucrose, 5 mM Tris-HCl and 1 mM MgCl2, pH 7.4) and homogenized with a polytron homogenizer (Kinematica, Basel, Switzerland) at 4°C and at speed 7 for 10 s. The homogenate was then centrifuged at 1000 g at 4°C for 10 min to remove the nuclei. The supernatant fraction was centrifuged at 35 000 g for 20 min at 4°C. The resulting pellet was resuspended in binding buffer B (50 mM Tris-HCl, 10 mM MgCl2 and 10 mM EGTA, pH 7.4) and frozen at −80°C until the assay. The protein concentration was measured using the bicinchoninic acid protein assay kit (Pierce Chemical, Rockford, IL, USA). Radioligand binding studies were performed using [125I]-(2-b-(4-hydroxyphenyl)-ethylaminomethyl)-tetralone ([125I]-HEAT; 2200 Ci mmol−1; PerkinElmer Life and Analytical Sciences, Boston, MA, USA). In brief, 20–100 μg of membrane protein from brain was incubated with [125I]-HEAT in a final volume of 250 μl of binding buffer B, in the presence or absence of competing drugs, for 60 min at 25°C. The incubation was terminated by addition of ice-cold buffer B and immediate filtration through Whatman GF/C glass fibre filters with a Brandel cell harvester (model-30; Brandel Inc., Gaithersburg, MD, USA). Each filter was collected, and the radioactivity was measured. Binding assays were always performed in duplicate. Nonspecific binding was defined as binding displaced by 10 μM phentolamine.
Determination of sperm content in male sexual organs and sperm motility
Mice were anaesthetized with 50 mg kg−1 sodium pentobarbitone and killed by decapitation. The testis, epidydimus and vas deferens were isolated and weighed. The sperm content of these tissues was measured following the procedure of Joyce et al. (1993). Briefly, tissues were homogenized with the polytron homogenizer on ice in PBS containing 0.05% Triton X-100 (Sigma, St Louis, MO, USA). The homogenate was diluted with phosphate-buffered saline and stained with 4% Trypan blue. The number of sperm nuclei was counted using a haemacytometer and the total number of sperm in each tissue was obtained. Daily sperm production (DSP) was estimated by dividing the number of sperm per gram of tissue by 4.84, as described previously (Joyce et al., 1993).
To assess sperm motility, fluid from the caudal epidydimus was diluted in Medium 199 containing 0.5% (wv−1) BSA. The diluted sample solution was incubated in the sample chamber (MICROSLIDES #HTR1099, VitroCom Inc., Mountain Lakes, NJ, USA), and sperm motility parameters, such as the percentage of motile sperm, percentage of progressive sperm, smoothed path velocity, straight line velocity, track velocity and amplitude of lateral head displacement, were determined using TOX IVOS (Hamilton Thorne Research, Beverly, MA, USA).
Number of ejaculated sperm and sexual behaviour
To determine the number of ejaculated sperm from male mice, the number of sperm in the female uterus was counted after overnight mating. To synchronize the female sexual cycle, 5 U of pregnant mare serum gonadotropin was injected intraperitoneally (i.p.) into sexually immature females (3–4 weeks old). Forty-eight hours after the injection, the mice were injected with 5 U of human chorionic gonadotropin and mated with α1-AR triple-KO male mice for 16 h. The formation of a vaginal plug and the number of ejaculated sperm in the uterus were then determined as described above. Typical sexual behaviour, such as sniffing, chasing and mounting, were monitored after mating for 2 h, as described previously (Ratnasooriya and Wadsworth, 1990; Ban et al., 2002). The total number exhibiting typical sexual behaviour, as an index of libido (number mated per number paired × 100), was estimated.
Mechanical responses
The thoracic aorta and mesenteric artery were isolated from anaesthetized animals and dissected free of excess fat and connective tissue. Each artery was helically cut into a section 15–20 mm in length and 1 mm in width. The intimal surface of each artery was gently rubbed with a moistened filter paper to remove the endothelium, the functional absence of which was confirmed by the lack of a relaxant response to acetylcholine (10 μM). Aortic or mesenteric artery preparations were suspended in a 20-ml organ bath filled with a normal Tyrode's solution (in mM: NaCl 158.3, KCl 4.0, CaCl2 2.0, MgCl2 1.05, NaH2PO4 0.42, NaHCO3 10.0 and glucose 5.6), kept at 36.5±0.5°C and bubbled with a mixture of 95% O2 and 5% CO2. To prevent the oxidation of noradrenaline (NA), L-ascorbic acid (10 μM) was added to the solution. The tension was monitored continuously and recorded isometrically by a force displacement transducer. Experiments were conducted in the presence of propranolol (1 μM), yohimbine (0.3 μM), desipramine (0.3 μM) and deoxycorticosterone acetate (10 μM), to block β1-/β2- and α2-adrenoceptors and to inhibit the neural and non-neural uptake of NA, respectively. To determine the responsiveness of the vas deferens to drugs, it was isolated, dissected and incubated as described above. An analysis of contractile function of the vas deferens was performed by applying electrical stimulation at frequencies of 2–32 Hz.
Measurement of BP and heart rate
The systolic BP (SBP) and heart rate (HR) were measured in conscious mice with a computerized tail-cuff system (BA-98A system; Softron Co., Tokyo, Japan) that determines SBP using a photoelectric sensor (Tanoue et al., 2002b). Mice were anaesthetized with sodium pentobarbitone (40 mg kg−1 i.p.) and a stretched intramedic PE-10 polyethylene catheter (Clay Adams, Parsippany, NJ, USA) was inserted into the right carotid artery. The catheter was connected to a pressure transducer (SPR-671, Millar Instruments, Houston, TX, USA), and the MAP was recorded on a PowerLab system (Bio Research Center, Nagoya, Japan). Propranolol (3 mg kg−1) was injected before the experiments to avoid any effects due to stimulation of β-adrenoceptors. To examine the pressor responses, drugs in a volume of 30 μl were administered through a catheter inserted into the right femoral or jugular vein as a bolus at 15–20 min intervals after ensuring that the MAP and HR had returned to their baseline levels.
Statistics
Data are expressed as the means±s.e. Statistical analysis was performed using analysis of variance followed by a post hoc comparison with Fisher's PLSD using Statview version 5.0 software (Concepts, Inc., Berkeley, CA, USA). Differences between groups were considered statistically significant when P<0.05.
Drugs
The following drugs were used: NA bitartrate (Wako-junyaku, Osaka, Japan); prazosin hydrochloride, phentolamine hydrochloride, (−)-phenylephrine hydrochloride, propranolol hydrochloride, yohimbine hydrochloride, desipramine hydrochloride, αβ-methylene ATP lithium salt and deoxycorticosterone acetate (Sigma, St Louis, MO, USA).
Results
Receptor binding and general characteristics
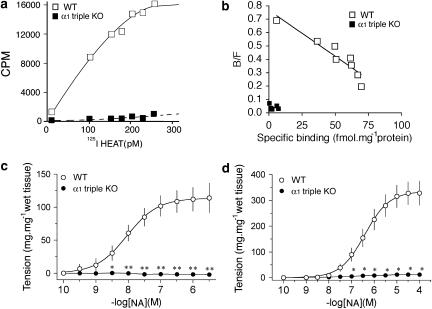
To confirm the disruption of all α1-adrenoceptor genes, a binding assay was performed using the cerebral cortex membrane from α1-AR triple-KO mice (Figures 1a and b). A saturation binding and Scatchard plot clearly indicated that the α1-adrenoceptor was completely absent in α1-AR triple-KO mice (Figures 1a and b). The maximum binding density of the cerebral cortex membrane from the wild-type (WT) mice was 102±26 fmol mg−1protein, while that from the α1-AR triple-KO mice was undetectable. α1-AR triple-KO mice survived, developed normally and grew for at least 1 year. Similar tissue weights were observed with α1-AR triple-KO and WT mice (Table 1).
Figure 1.
Characterization of the α1-adrenoceptor triple KO mouse (α1-AR triple-KO). (a and b) Density of the α1-adrenoceptor in cerebral cortex membrane from the α1-AR triple-KO mouse. (a and b) Saturation binding (a) and Scatchard plots (b) of [125I]-HEAT binding. (c and d) Drug responsiveness of vascular tissues in the α1-AR triple-KO mouse. Neither the aorta (c) nor the mesenteric artery (d) developed contractile force in response to NA in the α1-AR triple-KO mouse, while those from WT mice showed a contractile response in a dose-dependent manner. *P<0.05, **P<0.01 vs WT mice (WT). α1-AR triple-KO, α1-adrenoceptor triple KO; [125I]-HEAT, [125I]-(2-b-(4-hydroxyphenyl)-ethylaminomethyl)-tetralone; NA, noradrenaline; N.D., not detectable; WT, wild type.
Table 1.
Tissue weight at 8–15 weeks of age
| Body weight (g) | Heart weight (mg g−1) | Brain weight (mg g−1) | Liver weight (mg g−1) | Lung weight (mg g−1) | Testis weight (mg g−1) | Epidydimal weight with secretions (mg g−1) | Seminal vesicle weight with secretions (mg g−1) | |
|---|---|---|---|---|---|---|---|---|
| Wild type | 26±0.6 | 4.9±0.2 | 13.8±0.6 | 46.6±2.6 | 6.0±0.2 | 3.5±0.2 | 1.5±0.1 | 11.5±1.4 |
| α1-AR triple KO | 25±0.4 | 5.0±0.2 | 12.7±0.6 | 46.1±1.1 | 6.6±0.4 | 3.5±0.2 | 1.9±0.1 | 20.1±4.5 |
Abbreviations: α1-AR triple KO, α1-adrenoceptor triple KO; KO, knockout.
Values are expressed as the mean±s.e.m. (n=6 in each group).
Vascular response and BP
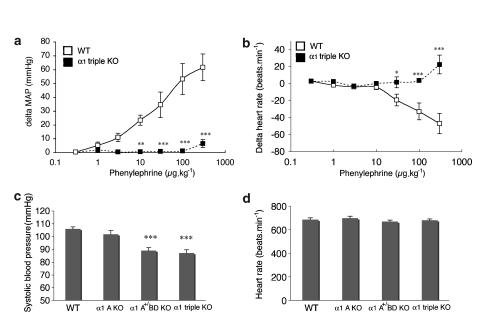
Next, we examined the contractile function of arteries, such as the aorta and mesenteric arteries, in response to NA (Figures 1c and d). Neither the aorta nor the mesenteric arteries from α1-AR triple-KO mice contracted in response to NA, while both the aorta and the mesenteric arteries from WT mice contracted in a concentration-dependent manner (Figures 1c and d). As in the in vitro experiments, the in vivo pressor response to phenylephrine, an α1-agonist, was dramatically lost in α1-AR triple-KO mice (Figures 2a and b). α1-AR triple-KO mice showed lower BP than WT mice (Figure 2b), and a reduced level of conscious BP was observed in both α1-AR triple-KO mice and the 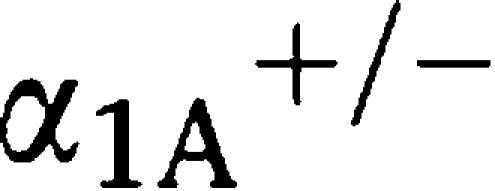
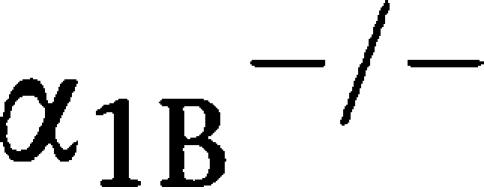
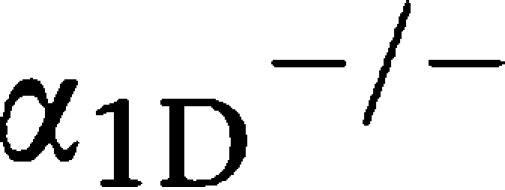 -adrenoceptor KO mice (
-adrenoceptor KO mice (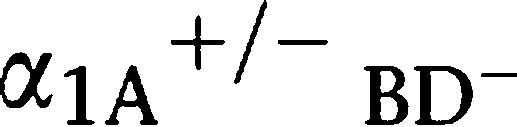 AR KO mouse) (Figure 2c). There were no differences in the HRs between the mice (Figure 2d).
AR KO mouse) (Figure 2c). There were no differences in the HRs between the mice (Figure 2d).
Figure 2.
Pressor response to vasoconstrictor agents in the α1-adrenoceptor triple KO mouse (α1-AR triple-KO). Drugs were intravenously injected through the jugular vein, and the mean arterial pressure (MAP) was monitored as described in the Methods section. (a and b) The changes in MAP (a) and HR (b) in response to phenylephrine are shown. The in vivo response of the MAP to a bolus injection of phenylephrine was not seen in α1-AR triple-KO mice. (c and d) Systolic BP and HR of conscious mice. *P<0.05, **P<0.01, ***P<0.001 vs WT mice. α1-AR triple-KO, α1-adrenoceptor triple KO; BP, blood pressure; HR, heart rate; KO. knockout; MAP, mean arterial pressure.
Male sexual function
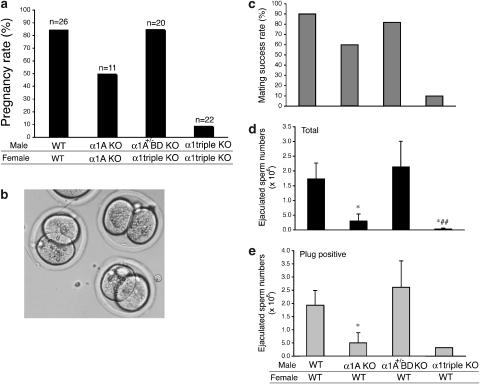
Although the α1-AR triple-KO mice developed normally, severe defects in the breeding activity were seen in α1A-AR KO mice. In order to confirm the presence of a defect in the breeding activity and analyse which α1-adrenoceptor contributes to this defect, we determined the pregnancy rate in each type of mouse. The pregnancy rate was reduced by 50% in α1A-AR KO mice (Figure 3a), and this reduction was dramatically enhanced in α1-AR triple-KO mice (Figure 3a). Since the pregnancy rate was similar when pairing WT male mice with WT females, and male 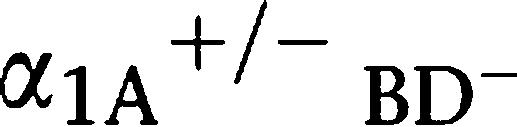 AR KO mice with female α1-AR triple-KO mice (Figure 3a), the reduction in the pregnancy rate was caused by male infertility.
AR KO mice with female α1-AR triple-KO mice (Figure 3a), the reduction in the pregnancy rate was caused by male infertility.
Figure 3.
Male sexual function in the α1-adrenoceptor triple KO mouse (α1α1-AR triple-KO). (a) Pregnancy rate of α1-AR triple-KO mice. An obvious reduction in the pregnancy rate was observed in females mated with male α1-AR triple-KO mice, while male 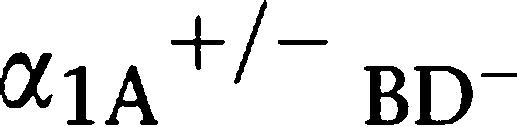 AR KO mice mated with female α1-AR triple-KO mice bred at the same rate as WT mice. α1A-AR KO mice showed a mild decrease in the pregnancy rate from that of WT. (b) In vitro fertilization using sperm and eggs from α1-AR triple-KO mice was successful and this is a typical picture of fertilized eggs taken 16 h after fertilization. (c–e) Number of sperm ejaculated from α1-AR triple-KO mice. (c) Mating success rate. The mating success rate was calculated by the number of vaginal plug-positive females divided by the total number of mating times. (d and e) Number of ejaculated sperm. Ejaculated sperm was determined by counting the sperm in the WT female uterus after a female mated with various male mice. Values are the averaged number of sperm in the uterus from plug-positive female mice (e) and the number of sperm from all mated female mice (d). *P<0.05 vs WT, ##P<0.01 vs
AR KO mice mated with female α1-AR triple-KO mice bred at the same rate as WT mice. α1A-AR KO mice showed a mild decrease in the pregnancy rate from that of WT. (b) In vitro fertilization using sperm and eggs from α1-AR triple-KO mice was successful and this is a typical picture of fertilized eggs taken 16 h after fertilization. (c–e) Number of sperm ejaculated from α1-AR triple-KO mice. (c) Mating success rate. The mating success rate was calculated by the number of vaginal plug-positive females divided by the total number of mating times. (d and e) Number of ejaculated sperm. Ejaculated sperm was determined by counting the sperm in the WT female uterus after a female mated with various male mice. Values are the averaged number of sperm in the uterus from plug-positive female mice (e) and the number of sperm from all mated female mice (d). *P<0.05 vs WT, ##P<0.01 vs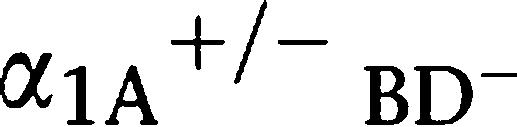 R KO. α1α1-AR triple-KO, α1-adrenoceptor triple KO; KO, knockout; WT, wild type.
R KO. α1α1-AR triple-KO, α1-adrenoceptor triple KO; KO, knockout; WT, wild type.
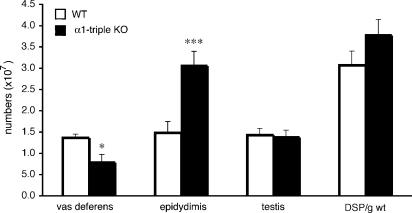
In order to address the underlying mechanisms of male infertility in α1A-AR KO and α1-AR triple-KO mice, we performed in vitro fertilization using sperm and eggs isolated from male α1-AR triple-KO and female mice. The in vitro fertilization was successful, and no obvious abnormalities were noted when comparing these mice with WT mice (Figure 3b), suggesting that the fertility of sperm from α1-AR triple-KO mice was normal. The sperm number in testis as well as DSP was identical in α1-AR triple-KO and WT mice (Figure 4). We then examined the motility of sperm from α1-AR triple-KO mice. All parameters of sperm motility, such as motile sperm, progressive sperm, smoothed path velocity and straight line velocity, were similar in sperm from α1-AR triple-KO, 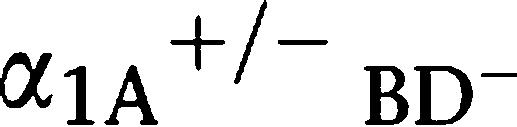 R KO, α1A-AR KO and WT mice (Table 2). Lack of sexual drive (libido) is another potential mechanism for a lower pregnancy rate, since the α1-adrenoceptors are associated with behavioural sensitization (Salomon et al., 2006). We counted typical sexual behaviour, such as sniffing, chasing and mounting, during mating for 2 h (Ratnasooriya and Wadsworth, 1990; Ban et al., 2002). There was no difference in the total number of typical sexual behaviours or the libido index between α1-AR triple-KO and WT mouse pairs (total number of typical sexual behaviours, 29±15 in α1-AR triple-KO mice and 37±12 in WT mice; libido index, 100% in α1-AR triple-KO mice and 100% in WT mice, n=7 pairs in each group).
R KO, α1A-AR KO and WT mice (Table 2). Lack of sexual drive (libido) is another potential mechanism for a lower pregnancy rate, since the α1-adrenoceptors are associated with behavioural sensitization (Salomon et al., 2006). We counted typical sexual behaviour, such as sniffing, chasing and mounting, during mating for 2 h (Ratnasooriya and Wadsworth, 1990; Ban et al., 2002). There was no difference in the total number of typical sexual behaviours or the libido index between α1-AR triple-KO and WT mouse pairs (total number of typical sexual behaviours, 29±15 in α1-AR triple-KO mice and 37±12 in WT mice; libido index, 100% in α1-AR triple-KO mice and 100% in WT mice, n=7 pairs in each group).
Figure 4.
Number of sperm in various organs from α1-adrenoceptor triple KO (α1-AR triple-KO) mice. The DSP was estimated by dividing the number of sperm per gram of tissue by 4.84, as described in the Methods section. *P<0.05, ***P<0.001 vs WT. α1-AR triple-KO, α1-adrenoceptor triple KO; DSP, daily sperm production; WT, wild type.
Table 2.
Sperm function
| Motile sperm (%) | Progressive sperm (%) | Path velocity (μm s−1) | Straight line velocity (μm s−1) | Curvilinear velocity (μm s−1) | Amplitude of lateral head displacement (μm) | |
|---|---|---|---|---|---|---|
| Wild type | 73±8 | 22±5 | 121±7 | 100±5 | 220±8 | 14.5±0.6 |
| α1A-AR KO | 78±1 | 15±2 | 119±3 | 92±2 | 210±5 | 14.5±0.4 |
| α1A+/−, BD-AR KO | 82±1 | 22±2 | 127±2 | 100±2 | 225±2 | 14.6±0.3 |
| α1-AR triple KO | 80±2 | 23±3 | 129±3 | 103±4 | 225±5 | 14.1±0.4 |
Abbreviation: α1-AR triple KO, α1-adrenoceptor triple KO; KO, knockout.
Values are expressed as the mean±s.e.m. (n=6 in each group).
Sperm ejaculation and vas deferens
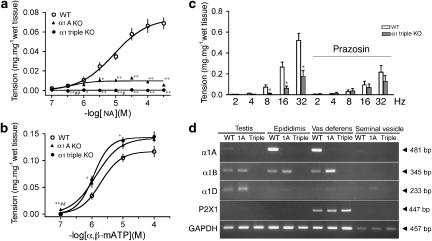
Since some α1-adrenoceptor antagonists are known to alter the male ejaculatory function (Debruyne, 2000; van Dijk et al., 2006) and this occurs more frequently with relatively selective α1A-adrenoceptor antagonists than with other drugs of this class, we analysed the ejaculatory function in α1A-adrenoceptor KO and α1-AR triple-KO mice. Female mice were mated with male α1-AR triple-KO, 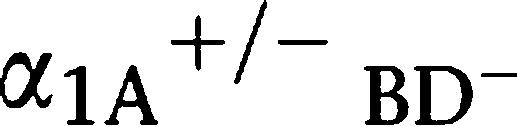 AR KO, α1A-AR KO or WT mice. The success rate of the mating, estimated by the presence of a vaginal plug the next morning, was decreased for male α1A-AR KO mice and further reduced for male α1-AR triple-KO mice (Figure 3c). Consistent with the mating success rate, the number of sperm in the female uterus was lower in α1A-AR KO mice and almost absent in α1-AR triple-KO mice, particularly when compared with that in WT mice (Figures 3d and e). A greater reduction in the sperm number was observed in the vas deferens of α1-AR triple-KO than in that of WT mice (Figure 4). This result suggests that the impaired transportation of sperm from the testis to the vas deferens is a potential mechanism of ejaculation dysfunction in α1-AR triple-KO mice. To analyse the underlying molecular mechanism(s) of ejaculatory dysfunction induced by disruption of the α1-adrenoceptor, we characterized the contractile function of vasa deferentia isolated from α1-AR triple-KO mice (Figure 5). Contractile tension of the vas deferens in response to NA was markedly decreased in α1A-AR KO mice, and this contraction was completely abolished in α1-AR triple-KO mice (Figure 5a), while contractile tension in response to α-β-methylene ATP (α-β-mATP) was enhanced in α1-AR triple-KO mice (Figure 5b). An attenuation of contractility was also observed in the electrically stimulated vas deferens (Figure 5c). This result is consistent with a decrease in the mating success rate of these animals as well as the reduced number of ejaculated sperm (Figures 3d and e). The expression profile of α1-adrenoceptors in male sexual organs showed that the α1A-adrenoceptor is dominant in both the epididymus and the vas deferens and that disruption of the α1A-adrenoceptor induces the upregulation of α1B- and α1D-adrenoceptors in these tissues (Figure 5d).
AR KO, α1A-AR KO or WT mice. The success rate of the mating, estimated by the presence of a vaginal plug the next morning, was decreased for male α1A-AR KO mice and further reduced for male α1-AR triple-KO mice (Figure 3c). Consistent with the mating success rate, the number of sperm in the female uterus was lower in α1A-AR KO mice and almost absent in α1-AR triple-KO mice, particularly when compared with that in WT mice (Figures 3d and e). A greater reduction in the sperm number was observed in the vas deferens of α1-AR triple-KO than in that of WT mice (Figure 4). This result suggests that the impaired transportation of sperm from the testis to the vas deferens is a potential mechanism of ejaculation dysfunction in α1-AR triple-KO mice. To analyse the underlying molecular mechanism(s) of ejaculatory dysfunction induced by disruption of the α1-adrenoceptor, we characterized the contractile function of vasa deferentia isolated from α1-AR triple-KO mice (Figure 5). Contractile tension of the vas deferens in response to NA was markedly decreased in α1A-AR KO mice, and this contraction was completely abolished in α1-AR triple-KO mice (Figure 5a), while contractile tension in response to α-β-methylene ATP (α-β-mATP) was enhanced in α1-AR triple-KO mice (Figure 5b). An attenuation of contractility was also observed in the electrically stimulated vas deferens (Figure 5c). This result is consistent with a decrease in the mating success rate of these animals as well as the reduced number of ejaculated sperm (Figures 3d and e). The expression profile of α1-adrenoceptors in male sexual organs showed that the α1A-adrenoceptor is dominant in both the epididymus and the vas deferens and that disruption of the α1A-adrenoceptor induces the upregulation of α1B- and α1D-adrenoceptors in these tissues (Figure 5d).
Figure 5.
Contractile dysfunction in the vas deferens from the α1-adrenoceptor triple KO mouse (α1-AR triple-KO). (a and b) Contractile tension in response to NA or α-β-mATP in the vas deferens from α1-AR triple-KO mice. Contractions in response to NA were not observed in the vas deferens from α1-AR triple-KO mice and were partially attenuated in α1A-AR KO mice, while contractions in response to α-β-mATP were enhanced in α1-AR triple-KO mice. (d) Expression levels of various receptor genes in sexual glands from α1-AR triple-KO mice. *P<0.05, **P<0.01, vs WT mice and #P<0.05, ##P<0.01 vs α1A-AR KO mice. α1-AR triple-KO, α1-adrenoceptor triple KO; α-β-mATP, α-β-methylene ATP; KO, knockout; NA, noradrenaline; WT, wild type.
Discussion
In the present study, we generated α1-AR triple-KO mice, in which the specific binding activity of the α1-adrenoceptor agonist was completely lost. The α1-AR triple-KO mice could survive, develop normally and grow for at least 1 year. The tissue weight and histological characteristics in the heart, kidney, aorta, testis, vas deferens and sperm (data not shown) were similar in the α1-AR triple-KO and WT mice. These results imply that α1-adrenoceptors and the subsequent signalling via α1-adrenoceptors are not necessary for survival.
In our previous study, we compared the in vitro contractile response of the aorta and mesenteric arteries as well as the in vivo pressor response of the MAP in α1B-AR KO, α1D-AR KO, and α1BD-AR double-KO mice (Hosoda et al., 2005a, 2005b). A greater reduction in the contractile force was observed in both the aorta and mesenteric arteries from α1BD-AR double-KO mice than in those from each of the subtype-specific α1-AR KO mice. However, a residual contraction, approximately 15–25% of the total tension in response to NA, was present in the tissues from the α1BD-AR double-KO mice (Hosoda et al., 2005a, 2005b). Similar to the in vitro results, the pressor response of MAP to α-adrenoceptor agonists was still evident in α1BD-AR double-KO mice (Hosoda et al., 2005a, 2005b). In the present study, neither the aorta nor the mesenteric arteries from α1-AR triple-KO mice developed tension in response to NA, while both the aorta and the mesenteric arteries from WT mice contracted in a concentration-dependent manner. As in in vitro experiments, the in vivo pressor response to phenylephrine, an α1-adrenoceptor agonist, was dramatically lost in α1-AR triple-KO mice. Our findings indicate that the disruption of all three α1-adrenoceptor subtypes can result in complete loss of the contractile function of vascular smooth muscle in response to catecholamine and the subsequent pressor response. These findings allow us to conclude that each of the α1-adrenoceptor subtypes, such as α1A/C-, α1B- and α1D-AR, is able to generate a physiological pressor response via α1-adrenoceptor signalling in the vascular system.
As described in a previous study, no obvious reduction in systolic BP was observed in α1A-AR KO mice, while a significant decrease in BP was detected in α1B- and α1D-AR KO mice (Cavalli et al., 1997; Tanoue et al., 2002b; O'Connell et al., 2003). Similar to α1A-AR KO mice, α1-AR triple-KO mice showed no additional reduction of conscious BP, in contrast to the 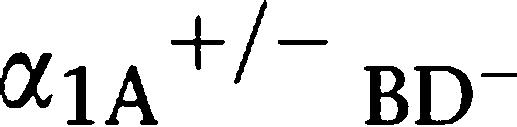 AR KO mouse. These results imply that the contribution of α1A-adrenoceptors to the maintenance of BP at the basal level is relatively small. In contrast to the BP, the pregnancy rate was reduced in α1A-AR KO mice, and this reduction was significantly enhanced in α1-AR triple-KO mice; this reduction in the pregnancy rate resulted from male infertility. These findings indicate that α1-adrenoceptor, particularly the α1A-subtype, plays an essential role in male fertility. To address the underlying mechanisms of male infertility in α1A-AR KO and α1-AR triple-KO mice, we performed in vitro fertilization and sperm motility analyses. All the results suggested that the fertilization ability of sperm and sperm motility are normal in male α1-AR triple-KO mice. The sperm number in testis as well as the DSP was identical in α1-AR triple-KO and WT mice. There was no difference in the total number of typical sexual behaviours between α1-AR triple-KO and WT mice during mating. Since no alterations in the histology of the testis and sperm of α1-AR triple-KO mice were detected when compared with those of WT mice (data not shown), the data from all the experiments strongly suggest that male infertility in α1A-AR KO and α1-AR triple-KO mice results from other mechanisms.
AR KO mouse. These results imply that the contribution of α1A-adrenoceptors to the maintenance of BP at the basal level is relatively small. In contrast to the BP, the pregnancy rate was reduced in α1A-AR KO mice, and this reduction was significantly enhanced in α1-AR triple-KO mice; this reduction in the pregnancy rate resulted from male infertility. These findings indicate that α1-adrenoceptor, particularly the α1A-subtype, plays an essential role in male fertility. To address the underlying mechanisms of male infertility in α1A-AR KO and α1-AR triple-KO mice, we performed in vitro fertilization and sperm motility analyses. All the results suggested that the fertilization ability of sperm and sperm motility are normal in male α1-AR triple-KO mice. The sperm number in testis as well as the DSP was identical in α1-AR triple-KO and WT mice. There was no difference in the total number of typical sexual behaviours between α1-AR triple-KO and WT mice during mating. Since no alterations in the histology of the testis and sperm of α1-AR triple-KO mice were detected when compared with those of WT mice (data not shown), the data from all the experiments strongly suggest that male infertility in α1A-AR KO and α1-AR triple-KO mice results from other mechanisms.
We observed an ejaculation dysfunction of the sperm concomitant with a lower rate of mating success in male α1A-AR KO mice, and this defect was enhanced in α1-AR triple-KO mice. All the findings indicate that the α1-adrenoceptor is required for normal sperm ejaculation and that the α1A-subtype plays a dominant role in sperm ejaculation. A recent clinical study indicates that α1-adrenoceptor antagonist-associated abnormal ejaculation may represent anejaculation rather than retrograde ejaculation (Hisasue et al., 2006; Hellstrom and Sikka, 2006). Although tamuslosin can facilitate rhythmic spike contractions that are not mediated by α1-adrenoceptors, in addition to having an antagonist action on α1-adrenoceptor-mediated contractions (Tambaro et al., 2005), all the results suggest that α1-adrenoceptors, particularly α1A-adrenoceptors, play an important role in ejaculatory function. Consistent with these clinical data, our present study showed that the contractile tension of the vas deferens induced by electrical stimulation as well as in response to NA was markedly decreased in α1A-AR KO mice and that this contraction was completely abolished in α1-AR triple-KO mice, while contractile tension in response to α-β-mATP was enhanced in α1-AR triple-KO mice. These results indicate that α1-adrenoceptors are required for normal contraction of the vas deferens and consequent sperm ejaculation. Our data also indicate that the α1A-subtype has an important role in all sexual functions and that the up-regulation of α1B- and α1D-subtypes can partially compensate for the dysfunction induced by blockade of the α1A-subtype. Since it is known that purinergic stimulation via the P2X1 receptor is necessary for normal ejaculation (Mulryan et al., 2000), it is probable that activation of purinergic nerves to stimulate the P2X1 receptor in association with sympathetic nerves to stimulate the α1-adrenoceptor is necessary for normal ejaculation.
In many previous studies the contractility in the vas deferens was analysed in rats using conventional antagonists, such as chlorethylclonidine, a relatively selective α1B-adrenoceptor antagonist, and RS 100329, an α1A-adrenoceptor antagonist (Han et al., 1987a, 1987b; Cleary et al., 2004). It has been shown that a different subtype of adrenoceptor, as well as other receptors, is involved in the contraction between the proximal and distal segments of the vas deferens and that this regulation by different receptors is also observed between tonic and phasic contractions (Westfall and Westfall, 2001; Cleary et al., 2003; Cuprian et al., 2005). Moreover, while the α1A-adrenoceptor can contribute to the contraction in the vas deferens via the sympathetic nerve in all species, including rats and mice, there may be considerable species differences with regard to the involvement of an additional subtype of adrenoceptor (Westfall and Westfall, 2001). It is known that contractile responses to endogenous nerve stimulation and exogenous agonists may be also mediated via a different subtype of receptor (Mallard et al., 1992; Guh et al., 1995). We analysed the contractility in one segment of the vas deferens (containing the proximal and distal segments) of mice, and it was difficult to separate the phasic and tonic contractions under our present experimental conditions. Thus, our results may be due to activation of the various subtypes of adrenoceptor as well as other receptors (Westfall and Westfall, 2001; Cleary et al., 2003; Cuprian et al., 2005). While some technical difficulties need to be overcome for a detailed analysis of the responses of the mouse vas deferens, additional studies are needed to elucidate the functional regulation of the vas deferens by α1-adrenoceptors.
Although abnormal ejaculation is frequently seen with tamuslosin and silodosin, which are relatively selective for the α1A-adrenoceptor compared to other drugs of this class, in clinical studies no obvious effect on ejaculation was detected with relatively non-selective α1-adrenoceptor antagonists, such as alfuzosin, doxazosin, prazosin and terazosin, (van Dijk et al., 2006). It is known that tamuslosin is not a fully surmountable (α1A-adrenoceptor antagonist in the human vas deferens (Furukawa et al., 1995; Noble et al., 1997). Furthermore, previous studies showed that tamuslosin may have an effect on subtypes of 5-hydroxytryptamine and dopamine receptors (Wyllie, 1999; van Dijk et al., 2006). Hence, the effects on ejaculation may be caused by alterations in the central nervous system rather than peripheral tissues (van Dijk et al., 2006). Thus, the abnormal ejaculation induced by tamuslosin as well as other α1A-adrenoceptor antagonists may be caused by effects other than α1-adrenoceptor blockade (Tambaro et al., 2005). Hence, further studies are needed to analyse the cause of the abnormal ejaculation induced by α1A-adrenoceptor antagonists.
In conclusion, we have demonstrated that α1-adrenoceptors, particularly α1A-adrenoceptors, are required for normal contraction of the vas deferens and consequent sperm ejaculation. The contractile dysfunction of the vas deferens induced by the loss of functioning α1A-adrenoceptors can explain the side effect observed in patients being treated with an α1A-adrenoceptor blocker. This information is important for the treatment of urinary symptoms induced by BPH as well as prostatitis, as most patients with these conditions are young adults.
Acknowledgments
We would like to express our deepest gratitude to Dr Paul C Simpson, Cardiology Division, San Francisco Veterans Affairs Medical Center, and the Cardiovascular Research Institute and Department of Medicine, University of California at San Francisco, for help with experiments using alpha1A-adrenoceptor knockout mice. This work was supported in part by research grants from the Scientific Fund of the Ministry of Education, Science, and Culture of Japan, the Ministry of Human Health and Welfare of Japan, the Japan Health Science Foundation, the NOVARTIS Foundation, the Suzuken Memorial Foundation, the Japan Heart Foundation/Novartis Grant for a Research Award on Molecular and Cellular Cardiology, the Takeda Science Foundation and the Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Abbreviations
- α-β-mATP
α-β-methylene ATP
- BPH
benign prostatic hyperplasia
- DSP
daily sperm production
- WT
wild type
Conflict of interest
The authors state no conflict of interest.
References
- ALLHAT Collaborative Research Group Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- Ban Y, Sato T, Nakatsuka T, Kemi M, Samura K, Matsumoto H, et al. Impairment of male fertility induced by muscarinic receptor antagonists in rats. Reprod Toxicol. 2002;16:757–765. doi: 10.1016/s0890-6238(02)00050-3. [DOI] [PubMed] [Google Scholar]
- Burcelin R, Uldry M, Foretz M, Perrin C, Dacosta A, Nenniger-Tosato M, et al. Impaired glucose homeostasis in mice lacking the alpha1b-adrenergic receptor subtype. J Biol Chem. 2004;279:1108–1115. doi: 10.1074/jbc.M307788200. [DOI] [PubMed] [Google Scholar]
- Cavalli A, Lattion AL, Hummler E, Nenniger M, Pedrazzini T, Aubert JF, et al. Decreased blood pressure response in mice deficient of the alpha1b-adrenergic receptor. Proc Natl Acad Sci USA. 1997;94:11589–11594. doi: 10.1073/pnas.94.21.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Cleary L, Slattery J, Bexis S, Docherty JR. Sympathectomy reveals alpha 1A- and alpha 1D-adrenoceptor components to contractions to noradrenaline in rat vas deferens. Br J Pharmacol. 2004;143:745–752. doi: 10.1038/sj.bjp.0705987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary L, Vandeputte C, Docherty JR. Investigation of postjunctional alpha1- and alpha2-adrenoceptor subtypes in vas deferens from wild-type and alpha(2A/D)-adrenoceptor knockout mice. Br J Pharmacol. 2003;138:1069–1076. doi: 10.1038/sj.bjp.0705137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn JN. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Eur Heart J. 1988;9 Suppl A:171–173. doi: 10.1093/eurheartj/9.suppl_a.171. [DOI] [PubMed] [Google Scholar]
- Cuprian AM, Solanki P, Jackson MV, Cunnane TC. Cholinergic innervation of the mouse isolated vas deferens. Br J Pharmacol. 2005;146:927–934. doi: 10.1038/sj.bjp.0706357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne FM. Alpha blockers: are all created equal. Urology. 2000;56:20–22. doi: 10.1016/s0090-4295(00)00744-5. [DOI] [PubMed] [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, et al. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foglar R, Shibata K, Horie K, Hirasawa A, Tsujimoto G. Use of recombinant alpha 1-adrenoceptors to characterize subtype selectivity of drugs for the treatment of prostatic hypertrophy. Eur J Pharmacol. 1995;288:201–207. doi: 10.1016/0922-4106(95)90195-7. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Rosario DJ, Smith DJ, Chapple CR, Uchiyama T, Chess-Williams R. Alpha 1A-adrenoceptor-mediated contractile responses of the human vas deferens. Br J Pharmacol. 1995;116:1605–1610. doi: 10.1111/j.1476-5381.1995.tb16380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino RD, Perez DM, Piascik MT. Recent advances in the molecular pharmacology of the alpha 1-adrenergic receptors. Cell Signal. 1996;8:323–333. doi: 10.1016/0898-6568(96)00066-6. [DOI] [PubMed] [Google Scholar]
- Guh JH, Chueh SC, Ko FN, Teng CM. Characterization of alpha 1-adrenoceptor subtypes in tension response of human prostate to electrical field stimulation. Br J Pharmacol. 1995;115:142–146. doi: 10.1111/j.1476-5381.1995.tb16331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Abel PW, Minneman KP. Alpha 1-adrenoceptor subtypes linked to different mechanisms for increasing intracellular Ca2+ in smooth muscle. Nature. 1987a;329:333–335. doi: 10.1038/329333a0. [DOI] [PubMed] [Google Scholar]
- Han C, Abel PW, Minneman KP. Heterogeneity of alpha 1-adrenergic receptors revealed by chlorethylclonidine. Mol Pharmacol. 1987b;32:505–510. [PubMed] [Google Scholar]
- Hellstrom WJ, Sikka SC. Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. J Urol. 2006;176:1529–1533. doi: 10.1016/j.juro.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Hisasue S, Furuya R, Itoh N, Kobayashi K, Furuya S, Tsukamoto T. Ejaculatory disorder caused by alpha-1 adrenoceptor antagonists is not retrograde ejaculation but a loss of seminal emission. Int J Urol. 2006;13:1311–1316. doi: 10.1111/j.1442-2042.2006.01535.x. [DOI] [PubMed] [Google Scholar]
- Hosoda C, Koshimizu TA, Tanoue A, Nasa Y, Oikawa R, Tomabechi T, et al. Two alpha1-adrenergic receptor subtypes regulating the vasopressor response have differential roles in blood pressure regulation. Mol Pharmacol. 2005a;67:912–922. doi: 10.1124/mol.104.007500. [DOI] [PubMed] [Google Scholar]
- Hosoda C, Tanoue A, Shibano M, Tanaka Y, Hiroyama M, Koshimizu TA, et al. Correlation between vasoconstrictor roles and mRNA expression of alpha1-adrenoceptor subtypes in blood vessels of genetically engineered mice. Br J Pharmacol. 2005b;146:456–466. doi: 10.1038/sj.bjp.0706325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce KL, Porcelli J, Cooke PS. Neonatal goitrogen treatment increases adult testis size and sperm production in the mouse. J Androl. 1993;14:448–455. [PubMed] [Google Scholar]
- Kawabe K, Yoshida M, Homma Y. Silodosin, a new alpha(1A)-adrenoceptor-selective antagonist for treating benign prostatic hyperplasia: results of a phase III randomized, placebo-controlled, double-blind study in Japanese men. BJU Int. 2006;98:1019–1024. doi: 10.1111/j.1464-410X.2006.06448.x. [DOI] [PubMed] [Google Scholar]
- Mallard NJ, Marshall RW, Sithers AJ, Spriggs TL. Separation of putative alpha 1A- and alpha 1B-adrenoceptor mediated components in the tension response of the rat vas deferens to electrical field stimulation. Br J Pharmacol. 1992;105:727–731. doi: 10.1111/j.1476-5381.1992.tb09046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC. Evidence for more than one type of post-junctional alpha-adrenoceptor. Biochem Pharmacol. 1982;31:467–484. doi: 10.1016/0006-2952(82)90147-2. [DOI] [PubMed] [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- Noble AJ, Chess-Williams R, Couldwell C, Furukawa K, Uchyiuma T, Korstanje C, et al. The effects of tamsulosin, a high affinity antagonist at functional alpha 1A- and alpha 1D-adrenoceptor subtypes. Br J Pharmacol. 1997;120:231–238. doi: 10.1038/sj.bjp.0700907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell TD, Ishizaka S, Nakamura A, Swigart PM, Rodrigo MC, Simpson GL, et al. The alpha(1A/C)- and alpha(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J Clin Invest. 2003;111:1783–1791. doi: 10.1172/JCI16100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, et al. Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest. 2006;116:1005–1015. doi: 10.1172/JCI22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnasooriya WD, Wadsworth RM. Impairment of fertility of male rats with prazosin. Contraception. 1990;41:441–447. doi: 10.1016/0010-7824(90)90043-u. [DOI] [PubMed] [Google Scholar]
- Rokosh DG, Simpson PC. Knockout of the alpha 1A/C-adrenergic receptor subtype: the alpha 1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci USA. 2002;99:9474–9479. doi: 10.1073/pnas.132552699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon L, Lanteri C, Glowinski J, Tassin JP. Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proc Natl Acad Sci USA. 2006;103:7476–7481. doi: 10.1073/pnas.0600839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng M, Cotecchia S, Schenk F. A behavioral study of alpha-1b adrenergic receptor knockout mice: increased reaction to novelty and selectively reduced learning capacities. Neurobiol Learn Mem. 2001;75:214–229. doi: 10.1006/nlme.2000.3965. [DOI] [PubMed] [Google Scholar]
- Tambaro S, Ruiu S, Dessi C, Mongeau R, Marchese G, Pani L. Evaluation of tamsulosin and alfuzosin activity in the rat vas deferens: relevance to ejaculation delays. J Pharmacol Exp Ther. 2005;312:710–717. doi: 10.1124/jpet.104.074740. [DOI] [PubMed] [Google Scholar]
- Tanoue A, Koba M, Miyawaki S, Koshimizu TA, Hosoda C, Oshikawa S, et al. Role of the alpha1D-adrenergic receptor in the development of salt-induced hypertension. Hypertension. 2002a;40:101–106. doi: 10.1161/01.hyp.0000022062.70639.1c. [DOI] [PubMed] [Google Scholar]
- Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, et al. The alpha(1D)-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest. 2002b;109:765–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk MM, de la Rosette JJ, Michel MC. Effects of alpha(1)-adrenoceptor antagonists on male sexual function. Drugs. 2006;66:287–301. doi: 10.2165/00003495-200666030-00002. [DOI] [PubMed] [Google Scholar]
- Westfall TD, Westfall DP. Pharmacological techniques for the in vitro study of the vas deferens. J Pharmacol Toxicol Methods. 2001;45:109–122. doi: 10.1016/s1056-8719(01)00144-7. [DOI] [PubMed] [Google Scholar]
- Wyllie MG.Alpha1-adrenoceptor selectivity: the North American experience Eur Urol 199936Suppl 159–63.discussion 65 [DOI] [PubMed] [Google Scholar]