Abstract
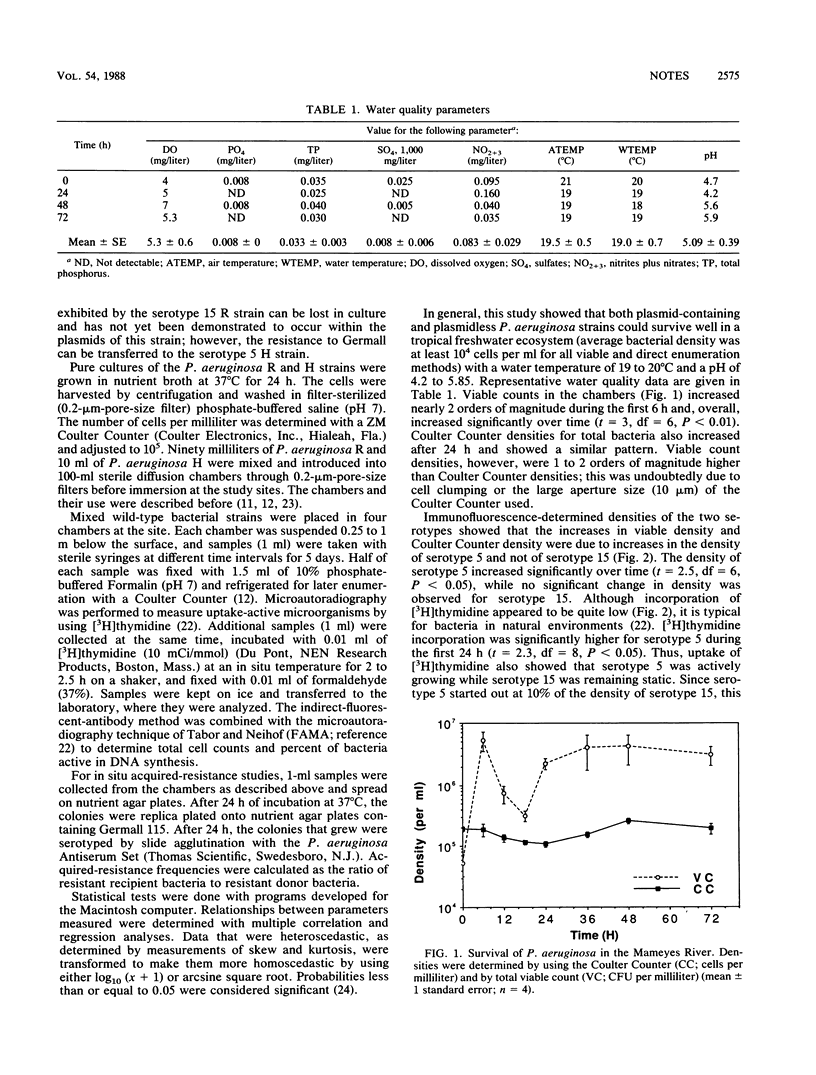
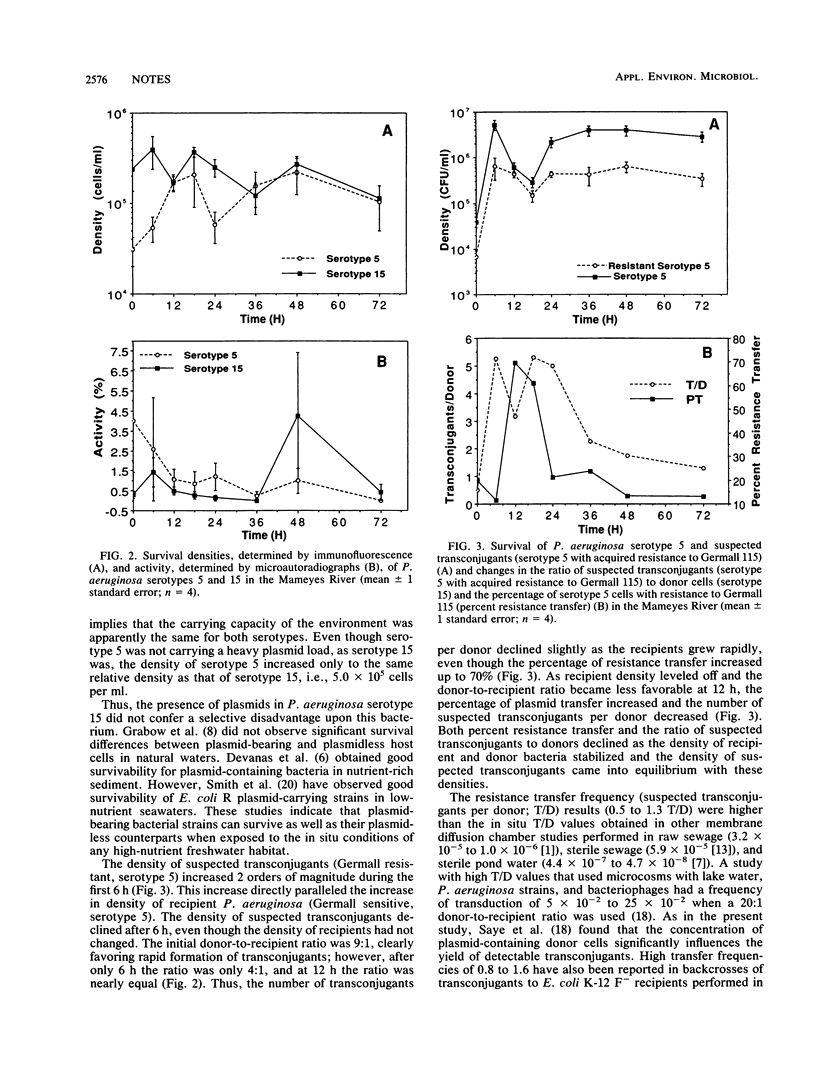
Two rare wild-type strains of Pseudomonas aeruginosa were mixed in membrane diffusion chambers and then introduced into a natural freshwater environment for 72 h. The plasmid-containing strain (R serotype 15) and the plasmidless strain (H serotype 5) had initial bacterial densities of 2 x 10(5) cells per ml. Samples collected from the chambers were analyzed for viable and direct counts and for acquired-resistance frequencies. Suspected transconjugant-to-donor ratios ranged from 0.5 to 1.3; transfer percentages ranged from 13 to 70%. [3H]thymidine uptake indicated DNA synthesis in both strains as well as in transconjugants. These studies indicate that rare wild-type bacterial strains with large plasmid loads can survive as well as can bacteria with low plasmid loads when exposed to the in situ conditions of a tropical freshwater habitat. These results also suggest that genetic modification of indigenous microbiota through conjugation or transformation is feasible when rare wild-type strains or genetically engineered microorganisms are released in large numbers in tropical aquatic ecosystems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altherr M. R., Kasweck K. L. In situ studies with membrane diffusion chambers of antibiotic resistance transfer in Escherichia coli. Appl Environ Microbiol. 1982 Oct;44(4):838–843. doi: 10.1128/aem.44.4.838-843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Aiba S. A perspective on the application of genetic engineering: stability of recombinant plasmid. Ann N Y Acad Sci. 1981;369:1–14. doi: 10.1111/j.1749-6632.1981.tb14172.x. [DOI] [PubMed] [Google Scholar]
- Mach P. A., Grimes D. J. R-plasmid transfer in a wastewater treatment plant. Appl Environ Microbiol. 1982 Dec;44(6):1395–1403. doi: 10.1128/aem.44.6.1395-1403.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo M. S., Cook W. L., Schlitzer R. L., Ward M. A., Wilson L. A., Ahearn D. G. Antibiograms, serotypes, and plasmid profiles of Pseudomonas aeruginosa associated with corneal ulcers and contact lens wear. J Clin Microbiol. 1986 Sep;24(3):372–376. doi: 10.1128/jcm.24.3.372-376.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H., Kettelhack C., Kettelhack M., Sonntag H. G., Keilich G., Brossmer R., Richards J., Kinzel V., Bäuerlein E., Pech H. Variation and adaptation of Pseudomonas aeruginosa toxicity to HeLa cells and fibroblasts. J Clin Microbiol. 1986 Sep;24(3):317–323. doi: 10.1128/jcm.24.3.317-323.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Roque C. M., Hazen T. C. Abundance and distribution of Legionellaceae in Puerto Rican waters. Appl Environ Microbiol. 1987 Sep;53(9):2231–2236. doi: 10.1128/aem.53.9.2231-2236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Mercado J., Hazen T. C. Comparison of four membrane filter methods for fecal coliform enumeration in tropical waters. Appl Environ Microbiol. 1987 Dec;53(12):2922–2928. doi: 10.1128/aem.53.12.2922-2928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saye D. J., Ogunseitan O., Sayler G. S., Miller R. V. Potential for transduction of plasmids in a natural freshwater environment: effect of plasmid donor concentration and a natural microbial community on transduction in Pseudomonas aeruginosa. Appl Environ Microbiol. 1987 May;53(5):987–995. doi: 10.1128/aem.53.5.987-995.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. R., Cabelli V. J. R-plasmid transfer frequencies from environmental isolates of Escherichia coli to laboratory and fecal strains. Appl Environ Microbiol. 1980 Oct;40(4):756–764. doi: 10.1128/aem.40.4.756-764.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. R., Farrell E., Dunican K. Survival of R+ Escherichia coli in sea water. Appl Microbiol. 1974 May;27(5):983–984. doi: 10.1128/am.27.5.983-984.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotzky G., Babich H. Fate of genetically-engineered microbes in natural environments. Recomb DNA Tech Bull. 1984 Dec;7(4):163–188. [PubMed] [Google Scholar]
- Tabor P. S., Neihof R. A. Improved microautoradiographic method to determine individual microorganisms active in substrate uptake in natural waters. Appl Environ Microbiol. 1982 Oct;44(4):945–953. doi: 10.1128/aem.44.4.945-953.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Collazo L., Schultz A. J., Hazen T. C. Survival of Candida albicans in tropical marine and fresh waters. Appl Environ Microbiol. 1987 Aug;53(8):1762–1767. doi: 10.1128/aem.53.8.1762-1767.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


