Abstract
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infections worldwide, yet no effective vaccine or antiviral treatment is available. Here we report the discovery and initial development of RSV604, a novel benzodiazepine with submicromolar anti-RSV activity. It proved to be equipotent against all clinical isolates tested of both the A and B subtypes of the virus. The compound has a low rate of in vitro resistance development. Sequencing revealed that the resistant virus had mutations within the nucleocapsid protein. This is a novel mechanism of action for anti-RSV compounds. In a three-dimensional human airway epithelial cell model, RSV604 was able to pass from the basolateral side of the epithelium effectively to inhibit virus replication after mucosal inoculation. RSV604, which is currently in phase II clinical trials, represents the first in a new class of RSV inhibitors and may have significant potential for the effective treatment of RSV disease.
Human respiratory syncytial virus (RSV) is the most important respiratory pathogen that causes lower respiratory tract infections, such as bronchiolitis and pneumonia, in infants and young children, resulting in up to 125,000 hospitalizations annually in the United States (3). The infants most at risk of severe disease are those under 6 weeks of age, those with bronchopulmonary dysplasia, congenital heart disease, or immunodeficiency, and those born prematurely. Hospital admission rates for these groups range between 5% and 30% (20, 25). The mortality rate among children admitted to hospital is approximately 3% for those with heart and lung problems and up to 1% for those without these risk factors (11, 25). In adults and the elderly, RSV pneumonia is increasingly recognized as a significant cause of morbidity and mortality, being associated with more than 17,000 deaths annually between 1991 and 1998 (9, 22). Among the hospitalized elderly, mortality can be as high as 10 to 20%, and among severely immunocompromised patients with RSV pneumonia, it can be on the order of 50 to 70% (10). There is therefore an urgent and unmet medical need for novel therapies to deal with infections caused by this virus.
The development of new therapeutics for RSV was recently reviewed (17, 19). Although research into the prevention and treatment of RSV infection has been ongoing for almost 40 years, vaccine development is difficult (8, 13), and to date, there is no clinically approved vaccine. The development of RSV vaccines for use in young infants has been complicated by reduced immune responses in this age group due to immunologic immaturity and the immunosuppressive effects of maternal antibodies. Passive immunization with the monoclonal antibody palivizumab (Synagis) has provided about 50% protection to high-risk children (21). These include infants born prematurely and those with congenital conditions. Because the antibody has to be given prophylactically and treatment is very expensive, its use is limited mainly to developed countries. The effectiveness of ribavirin (16), the only licensed antiviral small molecule against RSV, is questionable (24). Ribavirin has to be given by prolonged aerosol and has been shown to be potentially mutagenic and teratogenic. The need for an effective and safe treatment for RSV infection is paramount.
RSV, a pneumovirus of the paramyxovirus family, is an enveloped nonsegmented negative-strand RNA virus (6). The 15.2-kb genome has been sequenced fully and contains 10 mRNA species encoding 11 distinct proteins. The genome is encapsidated by the nucleocapsid (N) protein, which forms a helical nucleocapsid and protects the RNA from ribonucleases. The N protein is also associated with the viral polymerase, phosphoprotein, and M2-1 protein, which together form the transcriptase complex. The ribonucleoprotein (RNP) is essential for transcription, and naked RNA does not provide a template for the viral polymerase. As with all single-stranded RNA viruses, the virus does not have a proofreading mechanism during replication, resulting in a relatively high error rate and frequent mutations. Promising inhibitors targeting the fusion event were unsuccessful, partly because of the rapid emergence of resistant mutants mapping to the F gene (5). Therefore, an effective antiviral compound should ideally target essential genes of the replication complex, as these are often more highly conserved due to their functional role (19).
In this study, we describe the identification of RSV604, a potent RSV inhibitor, and outline the experiments performed to determine its mechanism of action (MOA). The results suggest that RSV604 targets the nucleocapsid protein and is active post-RSV infection. RSV604 is currently in phase II trials.
MATERIALS AND METHODS
Cells and viruses.
HEp-2 cells and RSV strains Long and B were obtained from the American Type Culture Collection (Manassas, VA). Vero cells were obtained from the European Collection of Cell Cultures (Porton Down, United Kingdom). The RSV RSS and A2 strains and pneumonia virus of mice strain 15 were gifts from A. Easton (University of Warwick, United Kingdom). Bovine RSV (Snook strain) was kindly supplied by G. Taylor (Institute of Animal Health, Compton, United Kingdom). Cells and virus stocks were grown as reported previously (4). Clinical isolates of RSV were obtained from G. Toms (University of Newcastle, United Kingdom), P. Rice (St. Georges Hospital, London, United Kingdom), J. De Vincenzo (University of Tennessee, Memphis, TN), and the Health Protection Agency (Colindale, London, United Kingdom).
Synthesis of compounds.
RSV604 and related compounds were synthesized as previously described (4). BMS-433771 (5) was synthesized in-house. Ribavirin was purchased from Sigma.
Antiviral assays.
XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide, disodium salt; Sigma] assays were performed to assess the protective effects of compounds on cell viability. Vero cells were used for the primary screen of 20,000 compounds, and HEp-2 cells were used for all subsequent work. Plates (96-well) were seeded with 4 × 103 cells per well in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). Cells were infected the next day with sufficient RSV (RSS strain) to produce an approximately 80% cytopathic effect after 6 days. Cells were incubated during this period in the presence or absence of serial dilutions of compounds. The viability of cells was assessed after 6 days by using XTT. Only living cells can reduce the tetrazolium salts into colored formazan products. Results were expressed as 50% effective concentrations (EC50s) or 50% cell cytotoxicity (CC50) values. Compounds were tested at an initial concentration of 10 μM and contained a concentration of 0.5% dimethyl sulfoxide (DMSO). To measure the growth inhibitory effects of compounds on cells, a cell control (no virus) assay was performed in parallel.
Effects on the expression of intracellular RSV antigen were evaluated by enzyme-linked immunosorbent assay (ELISA). HEp-2 cells were seeded in 96-well plates (5 × 103 cells per well in DMEM containing 10% FBS) for 24 h. Cells were infected at a multiplicity of infection (MOI) of approximately 0.02 and incubated in the presence or absence of serial dilutions of compound for 3 days. Cells were then fixed and permeabilized with 75% methanol-25% acetone and blocked with 2% nonfat milk (Marvel)-0.05% Tween. Plates were then incubated in the presence of a mouse anti-RSV monoclonal antibody (NCL-RSV3; Novocastra) followed by a rabbit anti-mouse horseradish peroxidase-labeled secondary antibody (DAKO). The plates were developed with O-phenylenediamine in the presence of hydrogen peroxide, and absorbance was measured at a wavelength of 650 nm using a plate reader.
Plaque reduction assays were carried out by infecting HEp-2 cell monolayers with 0.2 ml of 500 PFU/ml of RSV (RSS strain) per well of a six-well plate. After 2 h, cells were overlaid with DMEM containing 2% FBS and 0.6% agarose and compounds were added from a dilution series. Plates were incubated for 5 days and fixed with 10% formaldehyde. The agarose plugs were removed, and viral plaques were visualized by methylene blue staining or immunostaining. Plaques were counted, and results were expressed as percentages of virus replication compared with the plaques counted on wells infected in the absence of inhibitor.
Generation and sequencing of resistant viral mutants.
HEp-2 cells were infected with RSV strain RSS (MOI, 0.01) and grown in the presence of compound until the cytopathic effect reached approximately 80 to 90%. Virus was harvested and used to reinfect cells in a similar manner. A starting dilution corresponding to 0.5× EC50 was used for the first passage, and at each passage, the compound concentration was either kept constant or doubled until a resistant mutant was isolated and its resistance verified by cell ELISAs and plaque reduction assays, using a DMSO-passaged wild-type (WT) virus as a control. Resistant mutants were then plaque picked three times under agarose. Total RNA was prepared from infected cell pellets and reverse transcribed into cDNA according to the Thermoscript protocol (Invitrogen, Paisley, United Kingdom), using random primers. Overlapping 1-kb DNA fragments covering the whole RSV (RSS) genome were amplified using a high-fidelity DNA polymerase (ThermalAce; Invitrogen). PCR conditions were 94°C for 1 min followed by 30 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min. PCR products were purified and sequenced directly using a Beckman CEQ-2000 capillary sequencing system. Resistant RSV sequences were aligned against the RSS strain (GenBank accession number U39662).
Reverse genetics.
Reverse genetic experiments were carried out as previously described (7). A QuikChange II XL site-directed mutagenesis kit (Stratagene) was used to introduce the following changes into the RSV antigenome plasmid (7): A313G, A385T, C415A, and A385T with C415A. The base numbers refer to those for the N open reading frame (the initiating ATG bases are numbered 1 to 3).
Recovered recombinant virus was propagated in HEp-2 cells, tested against RSV604 and A-33903 by plaque reduction assays, and compared to WT virus. An M2-2 gene deletion RSV mutant (2) was also tested as a control. Each of the recombinant viruses was sequenced in its entirety to ensure that no other mutations were introduced.
HAE cell culture model.
Human airway epithelial (HAE) cells were isolated and cultured as previously described (26). Briefly, human nasal, tracheobronchial, and bronchial airway epithelial cells were isolated and seeded on collagen-coated permeable membrane supports. Once the cells were confluent, the apical medium was removed and cells were maintained at the air-liquid interface for approximately 4 to 6 weeks to allow epithelial differentiation. Cultures containing ciliated cells and transepithelial resistances of ≥300 Ω/cm2 were inoculated via the luminal surface with a recombinant RSV expressing green fluorescent protein (GFP), named rgRSV (14). Infection was monitored by visualization of GFP with a fluorescence microscope. Compounds at various concentrations were added to the basolateral medium concomitantly with the virus.
RESULTS
Identification of RSV604.
A selection of approximately 20,000 compounds from the Arrow Therapeutics corporate collection were screened in a single-concentration (10 μM), cell-based XTT assay to identify active compounds that inhibited any target involved in virus replication. Compounds identified in this way exhibited potential antiviral activity and were rescreened at various concentrations appropriate for each compound to calculate an EC50 or CC50 value, defined as a 50% reduction in virus- or compound-induced cell death, respectively.
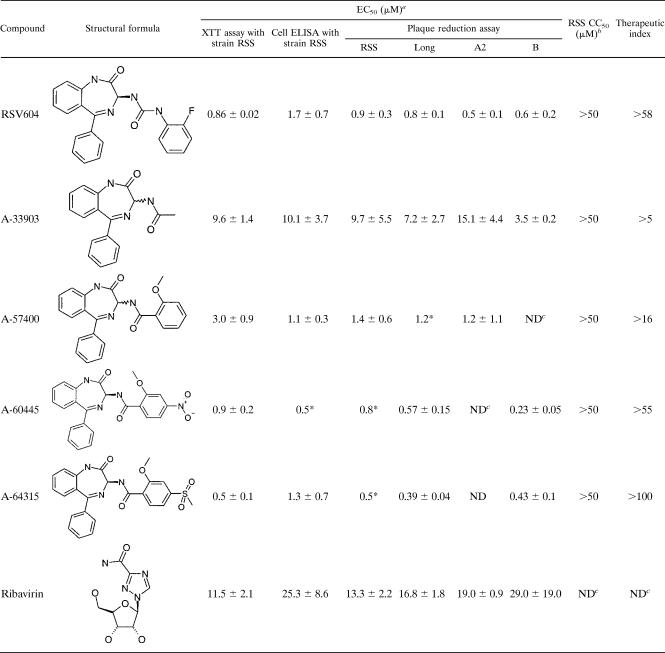
A number of compound classes with good therapeutic ratios (>50) were discovered. Selected compounds from each class were further tested against four different RSV strains in cell-based ELISAs and plaque reduction assays, which subsequently led to the selection of a lead compound, A-33903 (Table 1). This compound was chosen as a starting point for the synthesis of over 650 analogs. Continuous iterative cycles of synthesis, biological profiling, and modeling techniques ultimately resulted in the identification of the clinical candidate RSV604. Selected examples that illustrate the process of optimization are shown in Table 1. A detailed study of the structure-activity relationship for the chemical series was recently described by Carter et al. (4).
TABLE 1.
Biological profile of RSV nucleocapsid inhibitors
a Data are means ± SD for at least three independent experiments unless otherwise indicated (*, data are for one experiment). The XTT assay measures the ability to inhibit RSV-induced cell death. The cell ELISA measures the ability to reduce viral antigen synthesis in RSV-infected cells.
b Measured by XTT assay (n ≥ 2).
c ND, not determined.
Physical and chemical properties of RSV604.
The systematic name of RSV604 within the IUPAC nomenclature is (S)-1-(2-fluorophenyl)-3-(2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]di-azepin-3-yl)-urea, with the sum formula C22H17FN4O2 and a molar mass of 388.4 g/mol. The compound is a white powder which is stable at room temperature and has a melting point of 243 to 245°C (decomposition).
Profile of RSV604.
The first identified compound of the selected series, A-33903, a racemic mixture, showed moderate activity against four laboratory strains of RSV, and no associated toxicity was observed in the XTT assay with concentrations of up to 50 μM. It was approximately 2.5 times more active than ribavirin in cell ELISA (Table 1). An early derivative, A-57400, showed improved activity but also showed some growth inhibitory effects at 50 μM. Separation of the two isomers of A-33903 demonstrated that the antiviral activity was associated with the S enantiomer (15). Therefore, all subsequent compounds of this series tested were the isolated S enantiomers. The EC50 of the optimized compound RSV604 was determined to be 0.5 to 0.9 μM by plaque reduction assay. Similar activities were also observed in XTT assays and cell ELISA (Table 1). RSV604 exhibited a therapeutic ratio of >58 (limited by solubility) and was at least 15 times more potent than ribavirin.
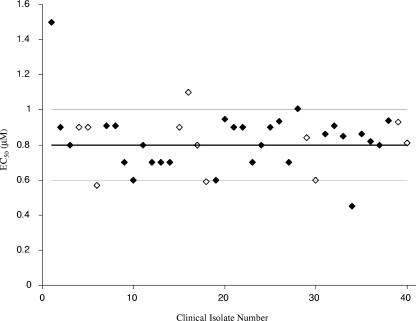
Increasing the MOI of RSV from 0.02 to 1.5 had only a slight effect on the EC50 of RSV604 (EC50 = 1.4 and 2.55 μM, respectively). RSV604 was also active against bovine RSV (EC50 = 0.3 μM in the plaque reduction assay), but no activity was observed against pneumonia virus of mice, parainfluenza virus type 1 (PIV1), PIV3, and human metapneumovirus (all with EC50s of >50 μM), with the latter being the human respiratory virus most closely related to RSV. Importantly, RSV604 was also equipotent against 40 clinical isolates of RSV, with an average EC50 of 0.8 ± 0.2 μM by plaque reduction assay (Fig. 1). Clinical isolates covered both the A and B subtypes, a 15-year span of infections, and the northern and southern hemispheres. None of the clinical isolates tested showed any resistance to RSV604, as demonstrated by the narrow range of EC50 values determined.
FIG. 1.
RSV604 EC50 values for 40 clinical isolates of RSV. The antiviral effect of RSV604 was investigated with 40 clinical isolates of RSV by plaque reduction assay, and EC50 values (μM) were calculated. The overall mean EC50 value (thick line) ± standard deviation (SD) (thin lines) of the 40 isolates is shown. Isolates 1 to 6 are from Hong Kong, isolates 7 to 18 are from Argentina, isolates 19 and 20 are from Qatar, isolates 21 to 30 are from Europe, and isolates 31 to 40 are from the United States. RSV-A strains are denoted by filled diamonds, and RSV-B strains are denoted by open diamonds.
MOA.
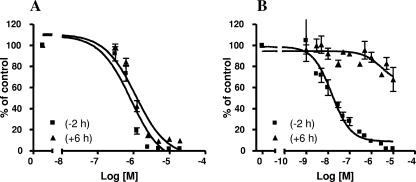
RSV604 was able to inhibit virus replication at similar levels when given prior to infection and at up to 6 h postinfection (Fig. 2A). These data strongly suggest that RSV604 acts at a step subsequent to adsorption and viral penetration. In contrast, BMS-433771, a known fusion inhibitor (5), was most active when given prophylactically or concurrently with the virus. It was less active when given at 6 h postinfection (Fig. 2B). The timing with which RSV604 was active is consistent with effects on viral transcription and/or RNA replication.
FIG. 2.
Antiviral effect of delayed addition of inhibitor. Dose-dependent anti-RSV activities of RSV604 (A) and the fusion inhibitor BMS-433771 (B) were determined in vitro by plaque reduction assay; compounds were added either 2 h prior to infection (−2 h) or at 6 h postinfection (+6 h). The mean of duplicate wells per compound dilution was used to calculate the percent plaque reduction compared to compound-free, virus-infected control wells. Results shown are the means and SD for four independent experiments.
The MOA of antiviral compounds is often examined through the generation of drug-resistant mutants. Mutants to RSV604 and A-33903 were isolated after multiple passages of virus with increasing concentrations of the compounds. An approximately eightfold increase in the cell ELISA-based EC50 was observed for the RSV604 mutant after eight passages compared to that for DMSO-passaged WT virus. This was confirmed by plaque assay, where a 10-fold resistance was observed. Three subsequent rounds of plaque picking at passage 8 (in the presence of 25 μM drug) resulted in a virus that had >40-fold resistance to RSV604 in comparison to the control virus in both the cell ELISA and plaque assay (Table 2). Resistance against A-33903 emerged at passage 5 (in the presence of 32 μM of compound), showing a twofold increase in the EC50 by cell ELISA compared to that for DMSO-passaged WT strain RSS. Resistance was confirmed at passage 6 (maximum concentration of 64 μM, or eight times the EC50) by cell ELISA and plaque reduction assay, showing about twofold and fourfold resistance, respectively. Three subsequent rounds of plaque picking at passage 6 resulted in a resistant virus that had a fourfold increased resistance to A-33903 in comparison to the control virus by plaque reduction assay (Table 2). The plaque-picked mutants were found to be cross-resistant to other compounds within the same chemical series (A-60445 and A-64315), indicating that the MOAs throughout the series are similar. Mutants did not show resistance to either ribavirin or a fusion inhibitor (A-57156), implying a different MOA for these compounds (Table 2).
TABLE 2.
Cross-resistance profiles of RSV604- and A-33903-resistant mutants
| Compound | RSV604 resistance
|
A-33903 resistance
|
||||
|---|---|---|---|---|---|---|
| EC50 (μM)a
|
Fold increase in resistanceb | EC50 (μM)a
|
Fold increase in resistanceb | |||
| DMSO-passaged WT | Resistant mutant | DMSO-passaged WT | Resistant mutant | |||
| RSV604 | 0.6 | 30.4 | 50.7 | 0.7 | 2.7 | 3.9 |
| A-33903 | 8.1 | >100 | >12.3 | 8.9 | 27.6 | 3.1 |
| A-60445 | ND | ND | ND | 0.4 | 1.3 | 3.3 |
| A-64315 | 0.4 | 16.9 | 42.3 | ND | ND | ND |
| A-57156c | <0.16 | <0.16 | ∼1 | <0.16 | <0.16 | ∼1 |
| Ribavirin | 20.7 | 17.6 | 0.85 | ND | ND | ND |
Determined by plaque reduction assay. A plaque-picked RSV604 mutant from passage 2 and a plaque-picked A-33903 mutant from passage 2 were tested for sensitivity to the indicated compounds. ND, not determined.
Increase in resistance of the mutant compared to its WT virus control.
Fusion inhibitor.
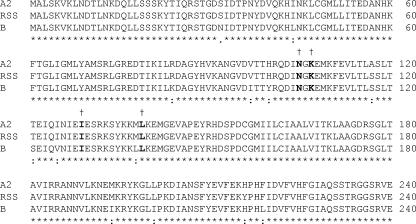
Sequencing of the complete genomes of the plaque-picked resistant viruses and the corresponding DMSO-passaged WT virus revealed mutational changes in the conserved N-terminal region of the nucleocapsid protein only (amino acids 105 to 139). The N gene of RSV was also sequenced from viruses obtained from the passages immediately prior to plaque picking. The amino acid substitutions identified were as follows: N105D in the A-33903-resistant RSV from passages 5 and 6 and the plaque-purified virus, K107N with I129L in the RSV604-resistant virus prior to plaque purification (passage 8), and I129L with L139I in the plaque-purified RSV604-resistant virus. The alignment of WT RSV sequences shows that these residues are conserved across RSV strains (Fig. 3). No additional mutations were identified in any of the other RSV genes.
FIG. 3.
Mapping of resistance to RSV604 to the nucleocapsid protein. A partial sequence alignment (from amino acids 1 to 240) for the N proteins from the human RSV strains A2 (Protein Data Bank [PDB] no. AAB59852.1), RSS (PDB no. NP044591.1), and subgroup B (PDB no. AAB82431.1) is given. Amino acid sequence identities in all three strains are denoted by asterisks. The positions of amino acid changes observed for the resistant mutants derived against RSV604 and A-33903 are given in bold and denoted with daggers.
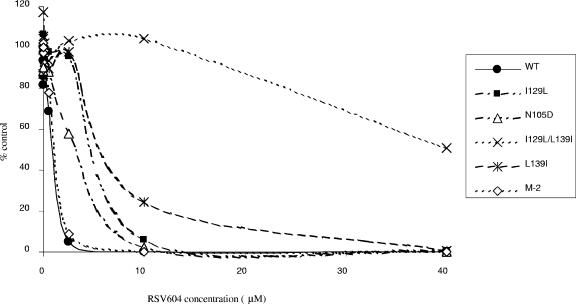
Reverse genetic experiments were carried out to confirm that these mutations were responsible for the observed resistance profile. The following base changes were individually introduced into the N gene of the A2 antigenome plasmid: A313G, A385T, C415A, and A385T with C415A. These make the following amino acid changes in the N protein: N105D, I129L, L139I, and I129L with L139I, respectively. Recombinant viruses were generated and analyzed. The resulting mutant viruses all showed resistance to RSV604 compared to the WT control (Fig. 4; Table 3). The levels of resistance were between 5-fold and 7-fold for the single mutants and >20-fold for the double mutant (Table 3). Each of the mutant genomes was fully sequenced, confirming the role of the mutations in the resistance profile. As an additional control, we also evaluated an attenuated RSV mutant in which the WT virus sequence had been modified by the introduction of point mutations into the M2-2 open reading frame (2). As expected, this virus (designated M-2) was equivalent to WT RSV in its sensitivity to RSV604 (Fig. 4). Growth curves were performed to assess the overall fitness of the mutant viruses. There was no significant difference in the replication of the mutant viruses compared to that of the WT (data not shown).
FIG. 4.
Sensitivities of WT and RSV mutant viruses to RSV604. Dose-dependent anti-RSV activities of RSV604 were determined by plaque reduction assay. Results are representative of three independent experiments. Mean EC50 values (± SD) for RSV604 against WT and mutant viruses can be found in Table 3.
TABLE 3.
Sensitivities of recombinant mutant viruses to RSV604
| Virus | RSV604 EC50 (μM)a | Fold increase in resistanceb |
|---|---|---|
| WT | 0.96 ± 0.04 | 1.0 |
| M2 | 1.14 ± 0.41 | 1.2 |
| N105D mutant | 4.78 ± 0.93 | 5.0 |
| I120L mutant | 6.03 ± 0.50 | 6.3 |
| L139I mutant | 6.70 ± 0.80 | 7.0 |
| I129L-plus-L139I mutant | >20 | >20 |
Data are means ± SD for three separate plaque reduction assays.
Compared to the WT virus control.
RSV604 inhibits RSV infection in a model of HAE.
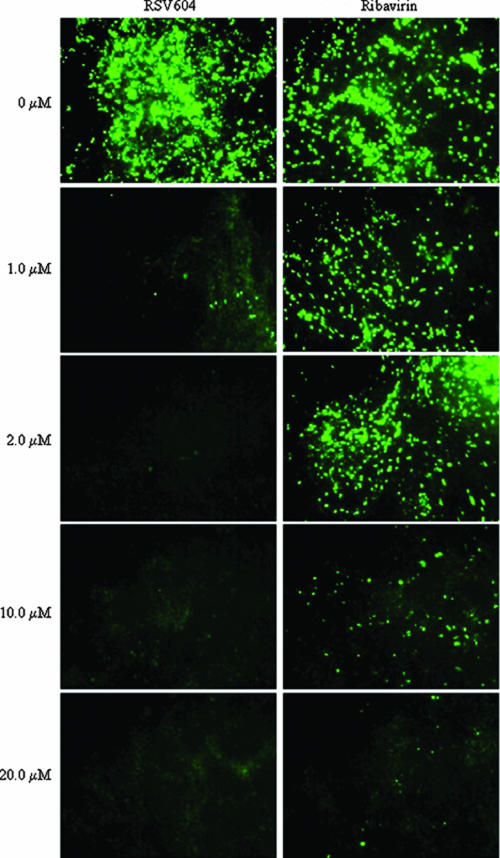
Due to the lack of small animal models of the HAE, in which human RSV infects and replicates efficiently (19), the antiviral activity of RSV604 was tested in a well-characterized in vitro model of differentiated HAE that recapitulates the morphology and physiology of the HAE in vivo. This model has previously been shown to support efficient infection, replication, and spread of a number of human respiratory viruses, including RSV (26). In this study, the apical surface of HAE was inoculated with a recombinant, GFP-expressing RSV (rgRSV; 106 PFU) and RSV604 was concomitantly added to the medium on the basolateral surface. RSV604 was able to block virus replication, as judged by the lack of green fluorescence, when given at a concentration of 2 μM. A dose of 1 μM inhibited virus replication approximately 50% (Fig. 5). Similar values were obtained with RSV604-treated RSV-infected HEp-2 cells (EC50 = 0.5 to 0.9 μM) (Table 1). Concentrations of 10 and 20 μM RSV604 completely blocked infection. RSV604 was at least 20-fold more active than ribavirin in inhibiting RSV replication in the HAE model (Fig. 5). Adding RSV604 (2, 10, or 20 μM) as late as 24 h postinfection resulted in a lack of green fluorescence, indicating inhibition of viral spread. No gross cytotoxicity, leakage of basolateral fluid to the apical surface, or alteration of cilium beat frequency was observed when HAE cultures were exposed to 20 μM RSV604 for 7 days.
FIG. 5.
Dose-dependent inhibition of RSV replication in an in vitro model of human ciliated epithelium. RSV replication was measured visually by the expression of GFP from the virus genome (21). The top two panels show control cultures in the absence of drug. Compound (RSV604 or ribavirin) was added to the basolateral medium concomitantly with the virus at the concentrations indicated.
DISCUSSION
Given the barriers to progression of an effective and safe RSV vaccine, the development of an orally active small-molecule drug which is virus specific and hence safe, potent, and effective is urgently needed.
We have identified a novel series of RSV inhibitors through the use of a well-selected library designed for antiviral screening containing only compounds that have the potential for further development. The current study shows that the lead compound from this series, RSV604, exhibits all of the above properties. RSV604 displays submicromolar activity against numerous clinical isolates of both the A and B subtypes of RSV, indicating a low probability of preexisting resistance mutations in the population. Unlike other promising inhibitors which mainly target the fusion event (19), RSV604 is active when given postinfection. Moreover, a significant reduction in viral spread was shown in an HAE cell model when RSV604 was given as late as 24 h postinfection.
The ability to rapidly generate resistant mutants in vitro, even at high concentrations of inhibitor (over 1,000× EC50), has posed a major problem for the development of an effective RSV drug in the past. The generation of fusion inhibitor-resistant mutants can be accomplished after several passages and may arise after only a single passage (5). In this study, the generation of mutants resistant to RSV604 was slow in comparison. It took eight passages in the presence of RSV604 before a resistant virus emerged. Without a suitable animal model of infection for the study of RSV replication inhibitors, we have been unable to characterize the replication of these resistant viruses in vivo. However, in vitro growth studies showed that the overall fitness of the mutant viruses engineered by reverse genetics was similar to that of the WT virus.
Sequencing revealed that the mutations in RSV604-resistant RSV mapped to the highly conserved nucleocapsid (N) protein. Reverse genetic experiments showed that when these mutations were introduced, alone or in combination, the resulting recombinant viruses were resistant to the effects of RSV604. This suggests that the N protein is a bona fide molecular target of RSV604. The N protein is a highly conserved major structural protein that is involved in encapsidation of the RNA genome and is essential for replication and transcription (6). The structures of the N proteins from two negative-strand viruses, vesicular stomatitis virus (12) and rabies virus (1), have recently been reported. In the case of these two viruses, the N protein is composed of two domains, with the single-stranded RNA molecule bound in a cavity at the interface. The two domains clamp down on the RNA and protect it from the surrounding environment. There is no crystal structure available for the RSV N protein, but further insight into RSV RNP complexes was recently provided by Tran et al. (23). The N protein assembles in an annular, multimeric complex of 10 or 11 subunits, with each subunit contacting six nucleotides. We are currently aiming to obtain a high-resolution (<3 Å) image of the structure of the RSV N protein to better predict what effects the mutations outlined in this study have on the protein and its interactions with other viral proteins. In particular, we will investigate the interaction between the N and P proteins. Murphy et al. suggested that amino acids 121 to 160 of the RSV N protein may be important for maintaining helical stability (18). The RSV604-resistant mutants had amino acid substitutions within this region which were shown to be important for resistance. With such a pivotal role in replication and transcription, it is highly likely that any effect on the N protein structure or function would be detrimental to the virus. This makes the N protein an excellent antiviral target.
In vivo studies with potential RSV inhibitors have been hindered by the lack of a suitable small animal model. The use of mice and cotton rats to study various aspects of RSV disease has been described previously; however, virus titers of up to 107 PFU are needed to infect these animals and the amount of virus recovered from the infected tissues is relatively low. This strongly suggests that replication in these animals is highly restricted. These models have been used successfully to study RSV monoclonal antibodies and potential fusion inhibitors whose effectiveness does not rely on active virus replication, but they are clearly unsuitable for the current study. To partially overcome this challenge, a three-dimensional HAE model was used in this investigation. This model was previously shown to support RSV replication specifically in ciliated cells after luminal inoculation, with exclusive apical shedding of progeny virions that are subsequently spread by the coordinated motion of the beating cilia. RSV604 administered to the basolateral medium was able to penetrate the multilayered epithelium of these cultures in sufficient amounts to efficaciously inhibit viral replication and spread. This indicates that the systemic delivery of this compound will be useful against luminal RSV infection.
A preclinical safety program was performed to assess the safety profile of RSV604 before commencing early studies in humans. It was concluded that RSV604 was well tolerated by all species tested, and an evaluation of the major organ systems in vivo demonstrated that there were no effects attributable to treatment. These promising preclinical data have enabled the study of the drug in phase I trials, where volunteers have been exposed to increasing quantities of RSV604. These studies show that the drug is well absorbed in humans, and the human pharmacokinetics support once-a-day dosing to achieve antiviral EC90 levels.
In conclusion, we have identified a novel, safe, and highly potent small-molecule inhibitor that targets the replication complex of RSV and constitutes the most promising candidate to date for the treatment of RSV disease in humans. Phase II clinical trials are under way to validate the effectiveness of RSV604 in treating RSV infection in the clinic.
Acknowledgments
P.L.C. and C.L. were supported by the NIAID Intramural Research Program.
P. L. Collins and Mark E. Peeples (Columbus Children's Research Institute, The Ohio State University) provided rgRSV. We thank G. Toms, P. Rice, J. De Vincenzo, and the HPA (London) for providing RSV clinical isolates.
Footnotes
Published ahead of print on 18 June 2007.
REFERENCES
- 1.Albertini, A. A. V., A. K. Wernimont, T. Muziol, R. B. G. Ravelli, C. R. Clapier, G. Schoehn, W. Weissenhorn, and R. W. H. Ruigrok. 2006. Crystal structure of the rabies virus nucleoprotein-RNA complex. Science 313:360-363. [DOI] [PubMed] [Google Scholar]
- 2.Bermingham, A., and P. L. Collins. 1999. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc. Natl. Acad. Sci. USA 96:11259-11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyce, T. G., B. G. Mellen, E. F. Mitchel, Jr., P. F. Wright, and M. R. Griffin. 2000. Rates of hospitalisation for respiratory syncytial virus infection among children in Medicaid. J. Pediatr. 137:865-870. [DOI] [PubMed] [Google Scholar]
- 4.Carter, M. C., D. A. Alber, R. C. Baxter, S. K. Bithell, J. Budworth, A. Chubb, G. S. Cockerill, V. C. L. Dowdell, E. A. Henderson, S. J. Keegan, R. D. Kelsey, M. J. Lockyer, J. N. Stables, L. J. Wilson, and K. L. Powell. 2006. 1,4-Benzodiazepine analogues as inhibitors of respiratory syncytial virus. J. Med. Chem. 49:2311-2319. [DOI] [PubMed] [Google Scholar]
- 5.Cianci, C., K. L. Yu, K. Combrink, N. Sin, B. Pearce, A. Wang, R. Civiello, S. Voss, G. Luo, K. Kadow, E. V. Genovesi, B. Venables, H. Gulgeze, A. Trehan, J. James, L. Lamb, I. Medina, J. Roach, Z. Yang, L. Zadjura, R. Colonno, J. Clark, N. Meanwell, and M. Krystal. 2004. Orally active fusion inhibitor of respiratory syncytial virus. Antimicrob. Agents Chemother. 48:413-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, P. L., R. M. Chanock, and B. R. Murphy. 2001. Respiratory syncytial virus, p. 1443-1485. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 7.Collins, P. L., M. G. Hill, E. Camargo, H. Grosfeld, R. M. Chanock, and B. R. Murphy. 1995. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc. Natl. Acad. Sci. USA 92:11563-11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, P. L., and B. R. Murphy. 2006. Vaccines against respiratory syncytial virus, p. 233-278. In P. Cane (ed.), Respiratory syncytial virus. Perspectives in medical virology, vol. 13. Elsevier BV Publishers, Amsterdam, The Netherlands. [Google Scholar]
- 9.Falsey, A. R., P. A. Hennessey, M. A. Formica, C. Cox, and E. E. Walsh. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352:1749-1759. [DOI] [PubMed] [Google Scholar]
- 10.Falsey, A. R., and E. E. Walsh. 2000. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 13:371-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fixler, D. E. 1996. Respiratory syncytial virus infection in children with congenital heart disease: a review. Pediatr. Cardiol. 17:163-168. [DOI] [PubMed] [Google Scholar]
- 12.Green, T. J., X. Zhang, G. W. Wertz, and M. Luo. 2006. Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. Science 313:357-360. [DOI] [PubMed] [Google Scholar]
- 13.Hall, C. B. 1980. Preventions of infections with respiratory syncytial virus: the hopes and hurdles ahead. Rev. Infect. Dis. 2:384-392. [DOI] [PubMed] [Google Scholar]
- 14.Hallak, L. K., D. Spillmann, P. L. Collins, and M. E. Peeples. 2000. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J. Virol. 74:10508-10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson, E. A., D. G. Alber, R. C. Baxter, S. K. Bithell, J. Budworth, M. C. Carter, A. Chubb, G. S. Cockerill, V. C. Dowdell, I. J. Fraser, R. A. Harris, S. J. Keegan, R. D. Kelsey, J. A. Lumley, J. N. Stables, N. Weerasekera, L. J. Wilson, and K. L. Powell. 2007. 1,4-Benzodiazepines as inhibitors of respiratory syncytial virus. The identification of a clinical candidate. J. Med. Chem. 50:1685-1692. [DOI] [PubMed] [Google Scholar]
- 16.Hruska, J. F., J. M. Bernstein, R. G. Douglas, Jr., and C. B. Hall. 1980. Effects of ribavirin on respiratory syncytial virus in vitro. Antimicrob. Agents Chemother. 17:770-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maggon, K., and S. Barik. 2004. New drugs and treatment for respiratory syncytial virus. Rev. Med. Virol. 14:149-168. [DOI] [PubMed] [Google Scholar]
- 18.Murphy, L. B., C. Loney, J. Murray, D. Bhella, P. Ashton, and R. P. Yeo. 2003. Investigations into the amino-terminal domain of the respiratory syncytial virus nucleocapsid protein reveal elements important for nucleocapsid formation and interaction with the phosphoprotein. Virology 307:143-153. [DOI] [PubMed] [Google Scholar]
- 19.Powell, K. L., and D. G. Alber. 2006. Development of antivirals against respiratory syncytial virus, p. 279-298. In P. Cane (ed.), Respiratory syncytial virus. Perspectives in medical virology, vol. 13. Elsevier BV Publishers, Amsterdam, The Netherlands. [Google Scholar]
- 20.Shay, D. K., R. C. Holman, R. D. Newman, L. L. Liu, J. W. Stout, and L. J. Anderson. 1999. Bronchiolitis-associated hospitalizations among US children 1980-1996. JAMA 282:1440-1446. [DOI] [PubMed] [Google Scholar]
- 21.The Impact-RSV Study Group. 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531-537. [PubMed] [Google Scholar]
- 22.Thompson, W. W., D. K. Shay, E. Weintraub, L. Brammer, N. Cox, L. J. Anderson, and K. Fukuda. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179-186. [DOI] [PubMed] [Google Scholar]
- 23.Tran, T. L., N. Castagne, D. Bhella, P. F. Varela, J. Bernard, S. Chilmonczyk, S. Berkenkamp, V. Benhamo, K. Grznarova, J. Grosclaude, C. Nespoulos, F. A. Rey, and J. F. Eleouet. 2007. The nine C-terminal amino acids of the respiratory syncytial virus protein P are necessary and sufficient for binding to ribonucleoprotein complexes in which six ribonucleotides are contacted per N protein protomer. J. Gen. Virol. 88:196-206. [DOI] [PubMed] [Google Scholar]
- 24.Ventre, K., and A. G. Randolph. 2004. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst. Rev. 2004:CD000181. [DOI] [PubMed] [Google Scholar]
- 25.Wang, E. E. L., B. J. Law, J. L. Robinson, S. Dobson, S. al Jumaah, D. Stephens, F. D. Boucher, J. McDonald, I. Mitchell, and N. E. MacDonald. 1997. PICNIC (Pediatric Investigators Collaborative Network on Infections in Canada) study of the role of age and respiratory syncytial virus neutralizing antibody on respiratory syncytial virus illness in patients with underlying heart or lung disease. Paediatrics 99:3.E9. [DOI] [PubMed] [Google Scholar]
- 26.Zhang, L., M. E. Peeples, R. C. Boucher, P. L. Collins, and R. J. Pickles. 2002. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells and without obvious cytopathology. J. Virol. 76:5654-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]