Abstract
Sec14p is an essential phosphatidylcholine/phosphatidylinositol transfer protein with a well-described role in the regulation of Golgi apparatus-derived vesicular transport in yeast. Inactivation of the CDP-choline pathway for phosphatidylcholine synthesis allows cells to survive in the absence of Sec14p function through restoration of Golgi vesicular transport capability. In this study, Saccharomyces cerevisiae cells containing a SEC14 temperature-sensitive allele along with an inactivated CDP-choline pathway were transformed with a high-copy-number yeast genomic library. Genes whose increased expression inhibited cell growth in the absence of Sec14p function were identified. Increasing levels of the Rho GTPase Cdc42p and its direct effector kinases Cla4p and Ste20p prevented the growth of cells lacking Sec14p and CDP-choline pathway function. Growth suppression was accompanied by an increase in large and multiply budded cells. This effect on polarized cell growth did not appear to be due to an inability to establish cell polarity, since both the actin cytoskeleton and localization of the septin Cdc12p were unaffected by increased expression of Cdc42p, Cla4p, or Ste20p. Nuclei were present in both the mother cell and the emerging bud, consistent with Sec14p regulation of the cell cycle subsequent to anaphase but prior to cytokinesis/septum breakdown. Increased expression of phosphatidylinositol 4-kinases and phosphatidylinositol 4-phosphate 5-kinase prevented growth arrest by CDC42, CLA4, or STE20 upon inactivation of Sec14p function. Sec14p regulation of phosphoinositide levels affects cytokinesis at the level of the Cdc42p/Cla4p/Ste20p signaling cascade.
The role of the Saccharomyces cerevisiae phosphatidylcholine (PC)/phosphatidylinositol (PI) transfer protein Sec14p as an essential regulator of Golgi apparatus-derived vesicular transport is well established (3-5, 10, 22, 31, 44, 52, 54, 58, 68). A set of five recessive mutations have been identified that allow cells to live in the absence of the normally essential SEC14. Inactivation of these genes bypasses the requirement for Sec14p in secretion from the Golgi apparatus and thus restores life to sec14 cells. Each of these “bypass sec14” genes codes for a protein involved in the regulation of phospholipid metabolism. These include the three enzymes of the CDP-choline pathway for PC synthesis, Cki1p, Pct1p, and Cpt1p; the phosphatidylinositol-4-phosphate (PI-4P) phosphatase Sac1p; and a member of the oxysterol binding protein family that also binds PI-4P, Kes1p (10, 17, 22, 26, 31, 48, 69). Experimental evidence points to a role for the PC and PI binding capacity of Sec14p to act as a regulator of phospholipid metabolic flux within the Golgi apparatus to ensure a secretion-competent lipid environment (10, 31, 41, 52, 58, 66-68).
Determination of the structure of Sec14p (55) revealed that the same protein fold, termed the CRAL-TRIO domain, is predicted to be present within several mammalian proteins, including the guanine nucleotide exchange factors Trio, Dbl and Dbs (2, 13, 29, 43, 50, 51, 56, 61, 64); several GTPase-activating proteins, including those for Arf and Rho family members as well as neurofibromin (mutations in which cause the common autosomal dominant disease neurofibromatosis) (2, 20, 50); the ATCAY protein, inherited mutations in which are the cause of cerebellar ataxia (Cayman type) (6, 46); and retinaldehyde and α-tocopherol binding proteins (1, 8, 27, 38, 39, 47). Roles for these Sec14p domain-containing proteins include cellular migration, transformation, neural transmission, and the visual cycle, suggesting that Sec14p domains may regulate functions in addition to Golgi apparatus-derived vesicular transport.
Since “bypass sec14” mutations allow yeast cells to transport vesicles from the Golgi apparatus independently of Sec14p function, we performed a genetic screen in a “bypass sec14” strain as a means to unmask new roles for Sec14p. In yeast, cell cycle progression is accompanied by polarized growth from the mother to the daughter cell. A key event mediating polarized growth is recruitment of the small Rho family GTPase Cdc42p to the bud site (1, 8, 27, 47, 72), where Cdc42p interacts directly with several proteins including the p21-activated kinases (PAKs) Ste20p and Cla4p. Cla4p and Ste20p in turn signal as yet poorly characterized effectors to direct cell growth toward the bud (7, 11, 12, 15, 19, 62, 65).
Data in this study reveal that the Cdc42p/PAK signaling pathway is responsive to Sec14p regulation of phosphoinositide metabolism, which in turn affects polarized cell growth. The effect on polarized cell growth by Sec14p did not appear to be due to an inability to establish cell polarity but instead was due to slowed progression through the cell cycle at a stage between anaphase and cytokinesis/septum breakdown.
MATERIALS AND METHODS
Yeast media, strains, and plasmids.
The rich medium used was yeast-peptone-dextrose (YPD; 10 g/liter Bacto yeast extract, 20 g/liter Bacto peptone, and 20 g/liter dextrose), and the minimal medium was synthetic dextrose (SD; 1.7 g/liter yeast nitrogen base without amino acids, 5 g/liter ammonium sulfate, 20 g/liter dextrose) containing the required nutritional supplements to complement strain auxotrophies and maintain plasmids. Bacto agar was added to the medium at 2% (wt/vol) prior to autoclaving to generate agar plates. Yeast strains were constructed using routine molecular and yeast genetic procedures; they are listed in Table 1.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source (reference) |
|---|---|---|
| CTY160 | MATaura3 his3 lys2 cki1 sec14-1(ts) | V. Bankaitis (3) |
| CTY159 | MATaura3 his3 lys2 kes1 sec14-1(ts) | V. Bankaitis (3) |
| CTY100 | MATaura3 his3 lys2 sac1-26 sec14-1(ts) | V. Bankaitis (3) |
| CTY1-1A | MATaura3 his3 lys2 sec14-1(ts) | V. Bankaitis (3) |
| CTY182 | MATaura3 his3 lys2 | V. Bankaitis (3) |
| DLY680 | MATα ura his leu trp cdc42-1(ts) | D. Lew (27) |
The 2 μm plasmids for expression of PIK1, STT4, and MSS4 were the kind gift of Scott Emr (Cornell University); CLA4 and CDC24 were from Erfei Bi (University of Pennsylvania); BOI1 was from Alan Bender (Indiana University); ARF1, ARF2, and ARL1 were from Gerald Johnston (Dalhousie University); and STE20 was from Peter Pryciak (University of Massachusetts). A kinase-dead version of STE20 (K649R) was made by site-directed mutagenesis using the QuikChange II site-directed mutagenesis kit from Stratagene according to the manufacturer's instructions and was confirmed by DNA sequencing. A 2 μm plasmid for expression of BEM1 was obtained from Daniel Lew (Duke University), as were low-copy-number plasmids for expression of green fluorescent protein (GFP)-Cdc42p, and GFP-Cdc12p. Plasmids were constructed using standard molecular techniques. Unless otherwise indicated, other yeast genes used were amplified from genomic DNA of strain W303a by PCR using primers 500 bp upstream and downstream of the open reading frame. DNA derived from PCR was sequenced to ensure polymerase fidelity and subcloned into low-copy-number (ARS/CEN) and high-copy-number (2 μm) yeast shuttle vectors.
Genetic screen.
Yeast with a temperature-sensitive SEC14 allele (sec14-1ts) and an inactivated choline kinase (cki1) gene (strain CTY160) (10) were transformed with a high-copy-number yeast genomic library at the permissive temperature of 25°C. Transformants were replica plated onto mimimal medium at both 25°C and 37°C, and suppressors of growth were identified by the absence of a yeast colony at 37°C. Approximately 20,000 transformants were screened. The average insert size was ∼3 kb, implying that the genome was saturated approximately threefold. However, as described in Results, not all genes capable of affecting growth were recovered from the library screen, implying that the library is likely not complete. Of the 20,000 transformants, 65 colonies were identified as unable to grow at 37°C. Library plasmids were isolated from yeast, amplified in Escherichia coli, restriction enzyme mapped to eliminate identical inserts, and retransformed into CTY160 to ensure that suppression was plasmid dependent. The retransformed CTY160 strains containing library plasmids were transformed with SEC14 on a low-copy-number plasmid or empty vector. Only those strains that could be rescued by the presence of SEC14 on a low-copy-number plasmid at 37°C were considered further.
Immunofluorescence and microscopy.
Fixed cells were resuspended in 100 μg/ml calcofluor white in phosphate-buffered saline. Cells were washed five times with phosphate-buffered saline and mounted on polylysine-coated slides. GFP-Cdc12p and GFP-Cdc42p were visualized in live cells using the GFP filter set fitted onto a Zeiss Axiovert 200M microscope using a Plan-Neofluor 100× oil immersion objective lens. Images were captured using a Zeiss AxioCam HR camera with Zeiss Axiovision (version 4.4) software.
Metabolic labeling.
PC synthesis through the CDP-choline and phosphatidylethanolamine methylation pathways was measured by labeling yeast cells with [14C]choline chloride or [3H]methionine, respectively, as determined previously (23, 36, 37).
Measurement of vesicular transport.
The invertase secretion index was determined as described previously (10, 22, 66, 70).
RESULTS
Identification of high-copy-number suppressors of growth of a “bypass sec14” strain.
SEC14 is an essential gene whose study has been facilitated by the use of a temperature-sensitive allele, sec14ts (10). Loss of Sec14p function is accompanied by an inability to transport vesicles from the Golgi apparatus (3, 4, 10). Yeast cells with an inactivated CDP-choline pathway for PC synthesis can bypass the requirement for Sec14p due to reestablishment of Golgi apparatus-derived vesicular transport (10, 22). To identify new proteins/processes that are regulated by Sec14p, the “bypass sec14” yeast strain CTY160 (sec14ts cki1) was transformed with a high-copy-number yeast genomic library to identify proteins that suppressed cell growth at the nonpermissive temperature for the sec14ts allele. Transformants whose growth defect at 37°C could be rescued by the presence of SEC14 alone, and whose growth was unaffected by the presence of both SEC14 and CKI1 at any temperature, had their plasmid DNA inserts sequenced.
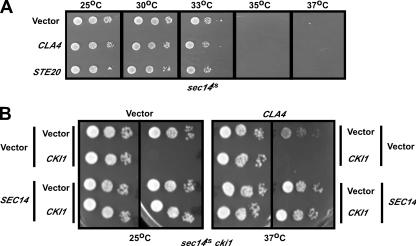
Of the plasmids that survived this analysis, two contained the CKI1 gene, coding for choline kinase, whose expression would directly reverse the bypass phenotype of the sec14ts cki1 strain. For plasmids containing more than one gene, potential suppressor genes were amplified from the yeast genome by PCR and individually subcloned into a high-copy-number yeast vector with transcription under the control of endogenous promoters. Seven genes from the library screen were confirmed to inhibit the growth of sec14ts cki1 cells (Table 2; Fig. 1A). Growth inhibition by each gene was prevented if a low-copy-number plasmid carrying SEC14 was transformed into these cells, indicating that growth inhibition was dependent on decreased function of Sec14p (Fig. 1C).
TABLE 2.
Genes isolated from the genetic screen that inhibit the growth of sec14ts cki1 cells
| Gene/ORFa | Description |
|---|---|
| STE20/YHL007C | Cdc42p-activated signal-transducing protein kinase that regulates polarized growth |
| ARK1/YNL020C | Serine/threonine protein kinase involved in regulation of the cortical actin cytoskeleton |
| CDC43/YGL155W | Beta subunit of geranylgeranyltransferase type I that has substrates important for morphogenesis |
| TIP1/YBR067C | Major cell wall mannoprotein with possible lipase activity |
| CLB5/YPR120C | B-type cyclin involved in DNA replication during S phase; activates Cdc28p |
| SEO1/YAL067C | Putative permease, member of the allantoate transporter subfamily of the major facilitator superfamily |
| GLC8/YMR311C | Regulatory subunit of protein phosphatase 1 Glc7p |
ORF, open reading frame.
FIG. 1.
Suppression of growth of sec14ts cki1 cells. (A) sec14ts cki1 cells expressing the indicated genes from a high-copy-number plasmid were grown to log phase in culture at 25°C. Equal numbers of cells were plated in 1:10 serial dilutions onto minimal medium and incubated at 25°C or 37°C. (B) CLA4 and CDC42 also suppress cki1 bypass of sec14ts. (C) sec14ts cki1 cells expressing the indicated genes from a high-copy-number plasmid were transformed with a low-copy-number plasmid containing SEC14 and grown to log phase in culture at 25°C. Equal numbers of cells were plated in 1:10 serial dilutions onto minimal medium and incubated at 25°C or 37°C.
The protein products of the isolated genes include two members of a complex that regulates polarized cell growth, Cdc43p and Ste20p. Ark1p is a serine/threonine protein kinase involved in the regulation of cortical actin cytoskeleton formation and endocytosis. Tip1p is a major cell wall protein of unknown function that contains a lipase motif with the potential to metabolize lipids. Clb5p is a B-type cyclin that activates the cyclin kinase Cdc28p to promote initiation of DNA replication. Glc8p is a regulatory subunit of the type 1 serine/threonine protein phosphatase Glc7p that regulates myriad cellular processes, and Seo1 is a putative permease of unknown function. We chose to focus on the role of Sec14p in the regulation of polarized cell growth because two genes that regulate this process, CDC43 and STE20, were recovered from our genetic screen and Sec14p homologues from other species are thought to regulate cell polarity through an unknown pathway(s).
Rational discovery of additional suppressor genes.
Cdc43p and Ste20p are part of the Cdc42p signal transduction pathway required for polarized cell growth and exit from mitosis (7, 8, 14, 16, 24, 46, 47, 50, 71). Membrane association of Cdc42p is required for its function, with Cdc43p prenylating Cdc42p to facilitate Cdc42p membrane association. Ste20p is a kinase that directly interacts with GTP-bound Cdc42p. Ste20p and a second, similar PAK, Cla4p, are downstream effectors of Cdc42p (7, 11, 12, 15, 19, 34, 62, 65). The guanine nucleotide exchange factor Cdc24p exchanges GDP for GTP to activate Cdc42p (7, 8, 12, 19, 57, 60), and signaling by the Cdc42p pathway is assisted by the scaffold protein Bem1p (7, 19, 27, 57). We determined the ability of each of these additional components of the Cdc42p signaling pathway to suppress the growth of sec14ts cki1 cells. CDC42 and CLA4 were strong suppressors of the growth of the sec14ts cki1 strain, while BEM1 and CDC24 were not (Fig. 1B). Growth suppression was dependent on loss of function of SEC14, as evidenced by the fact that transformation of cells with a low-copy-number plasmid carrying SEC14 prevented growth suppression (Fig. 1C).
To further assess the specificity of the growth defect upon increased expression of Cdc42p/PAKs to loss of function of both SEC14 and CKI1, we first transformed sec14ts cells with plasmids overexpressing CDC42, CLA4, or STE20. Increased expression of CLA4, STE20 (Fig. 2A), or CDC42 (data not shown) did not affect the nonpermissive temperature for growth of sec14ts cells. Second, we transformed sec14ts cki1 cells with low-copy-number CKI followed by low-copy-number SEC14 (or empty vectors), followed by high-copy-number CLA4 or an empty vector. Growth was not inhibited at the nonpermissive temperature for the sec14ts allele under any of these conditions. As expected, the sec14ts cki1 cells transformed with the CKI1 plasmid and empty vectors could no longer grow at the nonpermissive temperature for the sec14ts allele, while the same cells transformed with SEC14 alone, or both CKI1 and SEC14, grew at both 25°C and 37°C (Fig. 2B). The sec14ts cki1 cells containing a CLA4-overexpressing plasmid along with plasmid-borne SEC14 alone, or both CKI1 and SEC14, grew at both 25°C and 37°C. Consistent with the results shown in Fig. 2A, increased expression of CLA4 in sec14ts cki1 cells transformed with low-copy-number CKI1 did not rescue cell death at 37°C. Consistent with the results from the genetic screen, sec14ts cki1 cells containing empty vectors but a high-copy-number CLA4 plasmid exhibited reduced growth at 37°C (Fig. 2B). Growth inhibition by Cdc42p/PAKs is specific to simultaneous loss of both Sec14p function and PC synthesis through the CDP-choline pathway.
FIG. 2.
PAKs do not affect the growth of sec14ts cells. (A) The sec14ts strain was transformed with a high-copy-number plasmid expressing CLA4 or STE20 or with an empty vector, and equal numbers of cells were serially diluted and grown on solid medium at the indicated temperatures. (B) The sec14ts cki1 strain was transformed with a low-copy-number plasmid containing CKI1 or an empty vector, followed by a second transformation with a low-copy-number plasmid containing an empty vector or SEC14 and a high-copy-number plasmid containing CLA4 or an empty vector. Cells were grown to mid-log phase at 25°C, and equal numbers of cells were serially diluted, spotted onto solid medium, and grown at 25°C or 37°C.
To determine if it was the kinase-signaling properties of the Cdc42p/PAK complex, as opposed to assembly of the complex itself, that was responsible for growth suppression, a mutant version of Ste20p containing a point mutation (K649R) that renders its protein kinase inactive (45) was overexpressed in sec14ts cki1 cells. Ste20p(K649R) did not inhibit cell growth (Fig. 3A). Thus, it is the kinase signaling transduced by the Cdc42p/PAK pathway that inhibits cell growth when Sec14p function is diminished. We also assessed the specificity of the Cdc42p/PAK pathway versus other small-G-protein-regulated processes for inhibition of the growth of sec14ts cki1 cells. Increased expression of ARL1 or ARF2 did not affect the growth of sec14ts cki1 cells at either permissive or nonpermissive temperatures for the sec14ts allele, while increased ARF1 expression slightly inhibited growth at all temperatures (Fig. 3B). Inhibition of growth of sec14ts cki1 cells is not a general phenomenon associated with increased production of small G proteins in general.
FIG. 3.
PAK enzyme activity is required for growth suppression. (A) The sec14ts cki1 strain expressing STE20 or a version of STE20 (K649R) that renders the kinase inactive from a high-copy-number plasmid was grown to log phase in liquid culture at 25°C. Equal numbers of cells were plated in 1:10 serial dilutions onto minimal medium and incubated at 25°C or 37°C. (B and C) The sec14ts cki1 strain was transformed with 2μm plasmids for overexpression of ARL1, ARF1, ARF2, or BOI1. Cells were grown to log phase in liquid culture at 25°C. Equal numbers of cells were plated in 1:10 serial dilutions onto minimal medium and incubated at 25°C, 35°C, or 37°C.
There are a myriad of processes downstream of Cdc42p beyond the PAK pathway. One set of interactions is with Boi1p and Boi2p, two homologous proteins that also function in the maintenance of cell polarity. We transformed sec14ts cki1 cells with a high-copy-number plasmid expressing BOI1 to determine whether inhibition of cell growth was specific to activation of Cdc42p and its downstream PAKs Cla4p and Ste20p or was a general phenomenon associated with Cdc42p activation of any pathway that affects cell polarity. Increased expression of BOI1 did not affect the growth of sec14ts cki1 cells (Fig. 3C), implying that Sec14p affects Cdc42p/PAK function rather than all pathways immediately downstream of Cdc42p.
Cell viability and secretory competence.
Spo14p is the major PC phospholipase D in yeast; it hydrolyzes PC to produce phosphatidic acid and choline. SPO14 is normally not essential; however, loss of function of Spo14p in “bypass sec14” strains such as the sec14ts cki1 strain used here results in growth suppression followed by cell death due to reestablishment of the “bulk” Golgi vesicular transport block observed upon inactivation of SEC14 (58, 68). Several diagnostic assays were used to determine whether high-copy-number CDC42, CLA4, or STE20 was reestablishing a “bulk”' Golgi vesicular transport block or was preventing cell growth via a different mechanism.
Sec14p-regulated Golgi apparatus-derived vesicular transport is routinely measured by assessing the ability of sec14ts cells to secrete invertase after a shift to the nonpermissive temperature. All known “bypass sec14” mutations, including inactivation of the CDP-choline pathway for PC synthesis in sec14ts cki1 cells, result in reestablishment of invertase secretion. The invertase secretion index of sec14ts cki1 cells overexpressing CDC42, STE20, or CLA4 was assessed. Increased CDC42, STE20, or CLA4 expression did not decrease the ratio of invertase secreted from the Golgi apparatus at the nonpermissive temperature of 37°C compared to that for controls (Fig. 4A), indicating that invertase is successfully delivered from the Golgi apparatus to the plasma membrane and secreted.
FIG. 4.
Golgi apparatus-derived vesicular transport and viability of sec14ts cki1 cells. (A) Invertase secretion indices of wild-type, sec14ts, and sec14ts cki1 cells with or without high-copy-number CLA4, STE20, or CDC42 were determined. Error bars, standard errors of the means (n = 3). (B) Yeast cells were spotted in 1:10 dilutions of equal cell numbers onto a medium with or without 20 μg/ml calcofluor white. (C) Strains were grown to early log phase at 25°C, diluted to equal cell numbers, and shifted to 37°C. At the indicated time points, equal cell numbers were removed, and 1:10 serial dilutions were spotted onto plates and incubated at 25°C.
Sensitivity to calcofluor white is often observed in cells with secretory defects, cell wall defects, or stress signaling defects, as calcofluor white inhibits cell wall assembly (28). The calcofluor white sensitivity of secretory mutants is thought to arise from an impairment of the delivery of proteins required for cell wall synthesis. We observed that impairment of Sec14p function resulted in sensitivity to calcofluor white, since growth of the parental sec14ts strain on a medium containing calcofluor white lowered the restrictive temperature for growth to 33°C (Fig. 4B). The sec14ts cki1 bypass strain was not sensitive to calcofluor white, consistent with restoration of secretion from the Golgi apparatus. Increased expression of CLA4, STE20, or CDC42 did not affect the calcofluor white sensitivity of sec14ts cki1 cells, implying that growth inhibition does not occur via alterations in the delivery of cell wall-synthesizing proteins to the plasma membrane.
We also determined if the inability to grow in the absence of Sec14p function affected cell viability. For sec14ts cells grown at the nonpermissive temperature of 37°C, cell growth inhibition is associated with death over time. To assess if inhibition of cell growth in sec14ts cki1 cells due to overexpression of Cdc42p/PAKs resulted in cell death versus cell growth inhibition, cells were grown to log phase at the permissive temperature of 25°C and then shifted to the nonpermissive temperature of 37°C for various times. Equal cell densities of each culture were removed, and colony formation on solid medium at 25°C was determined. Only cells that remained viable at the restrictive temperature would form colonies at 25°C. The sec14ts strain lost significant viability after 16.5 h of growth at 37°C, and no viable cells were recovered after 27.5 h at 37°C (Fig. 4C). In contrast, sec14ts cki1 cells overexpressing CLA4, STE20, or CDC42 still formed colonies after 16.5 h at 37°C, with growth still obvious after 27.5 h at 37°C (Fig. 4C).
A trivial mechanism for inhibition of growth of the sec14ts cki1 strain by CDC42, STE20, or CLA4 could be through increased synthesis of PC (10, 22, 25, 48, 67). To assess if PC synthesis was altered, we determined the rates of labeled [14C]choline and [3H]methionine incorporation into PC by the CDP-choline and phosphatidylethanolamine methylation pathways, respectively, at 25°C and 37°C. There were no alterations in the ability to synthesize PC through the CDP-choline or phosphatidylethanolamine methylation pathway in cells overexpressing CDC42, STE20, or CLA4 compared to that for vector controls (data not shown).
The combined data are consistent with the Cdc42p/PAK signaling pathway inhibiting a Sec14p function that is not the delivery of “bulk” secretory cargo-containing vesicles.
Regulation of polarized cell growth.
Cell cycle delay in yeast cells is easily observed by morphological analysis, since the bud size increases as the cell cycle progresses (8, 9, 27). We observed that sec14ts cki1 cells with increased CDC42, STE20, or CLA4 expression grown at the nonpermissive temperature for the sec14ts allele accumulated cells with large buds and multibudded cells over time (Fig. 5A). We quantitated sec14ts cki1 cells grown at 37°C for 15 h according to bud size and observed that 23% of cells contained large buds, but this proportion more than doubled, to 50 to 55%, in cells expressing CLA4, STE20, or CDC42 (Fig. 5A and B). The number of cells with more than one bud also increased two- to threefold in cells expressing CLA4, STE20, or CDC42 (Fig. 5A and B). This is in contrast to results for vector controls, as well as both sec14ts and wild-type cells, where large budded cells represented only ∼20% of the population.
FIG. 5.
Suppressed strains accumulate large buds and multiple buds but have normal septin localization. (A) CTY160 (sec14ts cki1) cells containing a vector control or high-copy-number CLA4, STE20, or CDC42 were quantified according to bud size, and cells with different bud sizes are represented as percentages of the total population. (B) Calcofluor white staining of chitin at the bud neck. CTY160 cultures with a vector or overexpressing CLA4, STE20, or CDC42 were grown to log phase at 25°C, shifted to 37°C for 15 h, and then stained with calcofluor white. (C) CTY160 cells with or without high-copy-number CLA4, STE20, or CDC42 were grown to log phase at 25°C and then shifted to 37°C for 15 h. Cultures were fixed, and DNA was stained with 4′,6′-diamidino-2-phenylindole (DAPI) to visualize the nucleus. (D) CTY160 cells containing an empty vector or high-copy-number CLA4, STE20, or CDC42 were grown to log phase at 25°C and then shifted to 37°C for as long as 15 h. The septin Cdc12-GFP was localized to the bud neck in both suppressed and vector control strains at 37°C. Typical images are shown.
Chitin is deposited at the neck of a budding cell, eventually becoming a component of the new cell wall that is formed between the mother and daughter cells during cytokinesis (8, 9). When visualized by staining with calcofluor white, chitin normally appears as a narrow band located at the neck and is useful for distinguishing between a cell that is budding and two discrete cells that are adjacent to each other. Calcofluor white staining clearly indicated that in essentially all cases, apposed cells were a mother cell in the process of budding to produce a daughter cell (Fig. 5B).
An increase in the number of large budded cells is indicative of slowed cell cycle progression at G2/M. Nuclear DNA was stained to more precisely determine at which stage of G2/M the cells were affected. The large buds contained DNA, indicating that they had completed nuclear division/anaphase and were slowed at cytokinesis/septum breakdown (Fig. 5C).
Gross defects in actin cytoskeleton organization often reflect alterations in cell polarization. Cortical actin cytoskeleton organization is regulated by Cla4p and Ste20p in a Cdc42p-dependent manner (15). We assessed if cell polarity in general was affected by increased expression of CLA4, STE20, or CDC42 in sec14ts cki1 cells by visualizing the actin patches with rhodamine phalloidin. Although actin is partially depolarized in sec14ts cki1 cells (53), we did not observe any difference in actin patch distribution upon increased expression of CLA4, STE20, or CDC42 (data not shown).
A mitotic septin ring complex is laid down at the presumptive site of cytokinesis in a Cdc42p- and Cla4p-dependent manner (34, 62). We determined the localization of the septin Cdc12p fused with GFP in sec14ts cki1 cells overexpressing CLA4, STE20, or CDC42. GFP-Cdc12p was localized to the bud neck in a manner indistinguishable from that for wild-type cells at both 25°C and 37°C (Fig. 5D). The combined actin organization and GFP-Cdc12p localization data indicate that cell cycle inhibition lies downstream of the establishment of cell polarity and septin assembly, consistent with problems in cytokinesis or septum breakdown.
The “bypass sec14” gene KES1 is required for growth inhibition by Cdc42p/PAKs.
In the genetic screen performed here, the normally essential requirement for SEC14 was bypassed through inactivation of CKI1, which codes for one of the enzymes of the CDP-choline pathway for PC synthesis. Bypass of the essential function of SEC14 can also occur through inactivation of KES1, which codes for a member of the oxysterol binding protein family that also binds PI-4P, or of SAC1, which codes for a PI-4P phosphatase (10, 17, 22, 31, 48, 58, 68, 69).
Recently, Kes1p was found to aid in the proper localization of Cdc42p during polarized cell growth/cytokinesis (30), and consistent with this observation, we observed that increased CLA4, STE20, or CDC42 expression was unable to suppress the growth of sec14ts kes1 cells but did suppress the growth of sec14ts sac1 and sec14ts cki1 cells (Fig. 6A). Thus, proper localization of Cdc42p appears to be a requirement for inhibition of cell growth/cytokinesis when Sec14p function is decreased. To directly assess this, the location of GFP-Cdc42p was determined in sec14ts cki1 and sec14ts kes1 cells. At the nonpermissive temperature for the sec14ts allele, GFP-Cdc42p was found primarily at sites of polarized cell growth. Staining was generally a little more diffuse at these sites in sec14ts kes1 cells than in sec14ts cki1 cells (Fig. 6B). At 37°C, there was improper localization of GFP-Cdc42p in both sec14ts cki1 and sec14ts kes1 cells. In sec14ts cki1 cells, GFP-Cdc42p was still present on membranes, primarily at the cell surface, but was no longer localized to sites of polarized growth. GFP-Cdc42p was diffused throughout the cell in sec14ts kes1 cells. The data indicate that GFP-Cdc42p location is affected by inactivation of Sec14p function. GFP-Cdc42p still localizes to the cell periphery in cells lacking Sec14p and Cki1p function but is not polarized, while in cells lacking Sec14p and Kes1p function, GFP-Cdc42p is diffused throughout the cells and no longer associates with the cell surface.
FIG. 6.
Cdc42p/PAK does not suppress the growth of cells lacking KES1. (A) Strains CTY100 (sec14ts sac1) and CTY159 (sec14ts kes1) were transformed with multicopy CLA4, STE20, or CDC42. Strains were grown to log phase, and equal cell numbers were spotted in 1:10 serial dilutions onto minimal medium plates at 25°C and 37°C. (B) sec14ts cki1 or sec14ts kes1 cells were transformed with a low-copy-number plasmid encoding GFP-CDC42. Cells were grown to mid-log phase at 25°C, and a subset of these cells were shifted to 37°C for 1 h prior to imaging of live cells. Typical images are shown.
Phosphoinositide regulation of Cdc42p/PAK inhibition of cell growth.
Sec14p is a regulator of phosphoinositide levels in S. cerevisiae. The levels of PI-4P and PI-4,5P2 are decreased by 50% in sec14ts and sec14ts cki1 cells grown at the nonpermissive temperature (21, 53). To determine if the reductions in phosphoinositide levels due to decreased Sec14p function are responsible for Cdc42p/PAK inhibition of the cell cycle at cytokinesis, we transformed sec14ts cki1 cells with plasmids that result in increased expression of the PI 4-kinases Pik1p and Stt4p, as well as the PI-4P 5-kinase Mss4p. Increased Pik1p, Stt4p, or Mss4p expression alleviated the growth arrest due to the presence of overexpressed CLA4, STE20, or CDC42 when Sec14p function was reduced by the growth of cells at the nonpermissive temperature for the sec14ts allele (Fig. 7A). The regulation of phosphoinositide levels by Sec14p controls Cdc42p/PAK progression through cytokinesis.
FIG. 7.
Phosphoinositides relieve growth suppression by Cdc42p/PAKs. (A) sec14ts cki1 cells (CTY160) transformed with 2μm plasmids expressing CLA4, STE20, or CDC42 were transformed with a high-copy-number plasmid containing PIK1, STT4, or MSS4 or with an empty vector and then grown to log phase in liquid culture at 25°C. Equal cell numbers were plated in 1:10 serial dilutions onto minimal medium plates and incubated at 25°C or 37°C. (B) The cdc42ts strain was transformed with high-copy-number plasmids expressing PIK1, STT4, MSS4, SEC14, or an empty vector and was grown to log phase in liquid culture at 25°C. Equal cell numbers were plated in 1:10 serial dilutions onto minimal medium plates and incubated at 25°C, 35°C, or 37°C.
To order the events associated with the regulation of the Cdc42/PAK pathway by phosphoinositides, we overexpressed PIK1, STT4, MSS4, and SEC14 in a yeast strain containing a temperature-sensitive CDC42 allele (cdc42ts). The increased expression of these genes did not affect the growth of the cdc42ts strain at 25°C, 35°C, or 37°C (Fig. 7B), suggesting that phosphoinositides regulate either Cdc42p itself or a process upstream of Cdc42p.
DISCUSSION
This study has identified an additional role for Sec14p regulation of phospholipid metabolism beyond its well-characterized effects on Golgi apparatus-derived vesicular transport. Sec14p regulation of phosphoinositide levels in S. cerevisiae regulates cell cycle progression by way of the Cdc42p/Cla4p/Ste20p signaling cascade. This is subsequent to the establishment of cell polarity and affects efficient progression through cytokinesis/septum breakdown.
These findings are consistent with previous observations regarding Sec14p homologues in other organisms. Inactivation of the Sec14p homologue from Schizosaccharomyces pombe, spo20, results in defects in both Golgi secretory function and completion of cytokinesis (42). As well, the Sec14p homologues in the yeasts Yarrowia lipolytica and Candida albicans are essential for filamentous growth during mycelial growth mode (35, 40). Mycelial growth is a form of polarized cell growth that is highly invasive and is a factor that determines the infectivity of fungal parasites. A role for Sec14p-like proteins in polarized cell growth has also been observed with an Arabidopsis thaliana mutant lacking a Sec14p related protein, AtSfh1p, where root hairs possessed high frequencies of multiple growing tips (63).
A general role for PI transfer proteins in the regulation of cytokinesis is emerging, since a structurally distinct class of PI transfer proteins has also been determined to regulate both Golgi apparatus-derived vesicular transport and cytokinesis. The Drosophila PI transfer protein Giotto is required for mitotic and meiotic cytokinesis, and this is accompanied by a decrease in the level of trafficking of Golgi apparatus-derived vesicles (18), while the mammalian PI transfer protein Nir2 has been demonstrated to regulate both Golgi vesicular transport (32) and cytokinesis (33, 59). It is abundantly clear that a conserved function of PI transfer proteins of both structural classes is the regulation of Golgi apparatus-derived vesicular transport and cell division at the level of cytokinesis. The current study has advanced our understanding by determining that in S. cerevisiae the regulation of phosphoinositide metabolism by Sec14p controls cell cycle progression and that the Cdc42p/PAK signaling cascade is responsive to Sec14p-mediated changes in phosphoinositide metabolism. Whether the Cdc42p/PAKs are the sole process regulating cell fission by Sec14p control of phosphoinositide metabolism is an open question.
Increased expression of CDC42, CLA4, or STE20, but not of upstream activating components of this signaling pathway such as CDC24 or BEM1, prevented efficient cell cycle progression when Sec14p function was compromised. This implies that the phosphoinositide-responsive process is likely at or below the level of Cdc42p/PAKs. However, increased expression of the yeast PI 4-kinase STT4 or PIK1, or of the PI-4P 5-kinase MSS4, did not rescue the growth of cdc42ts cells, consistent with phosphoinositides regulating the function of the Cdc42p/PAK pathway at the level of Cdc42p or above. Combined, the results imply that phosphoinositides regulate Cdc42p function. In mammalian cells there is a link between phosphoinositide levels and Cdc42p function. The Wiskott-Aldrich syndrome protein (WASP) stimulates assembly of the actin cytoskeleton by the Arp2/3 complex, and WASP is synergistically activated through binding to both Cdc42 and PI-4,5P2 (49). However, determining precisely how regulation of phosphoinositide levels by Sec14p controls Cdc42p function will require further study. Other studies with yeast on the regulation of Cdc42p indicate that an on/off cycle of GTP loading, hydrolysis of GTP for subsequent release, and reloading of GTP is required for cytokinesis to proceed (27). Thus, either inactivation of the Cdc42p/PAK pathway or its constitutive activation can result in inefficient cytokinesis. Our current study is consistent with this model.
Previous to this work, the major defect observed upon inactivation of Sec14p was an inability to transport vesicles from the Golgi apparatus. Using a yeast strain with a temperature-sensitive allele of SEC14 along with a second mutation in the CDP-choline pathway for the synthesis of PC that results in the ability of cells to bypass the requirement for Sec14p in secretion from the Golgi apparatus, we uncovered a new role for Sec14p in S. cerevisiae. The regulation of phosphoinositide levels by Sec14p affects cell cycle progression at the level of cytokinesis by way of the Cdc42p/PAK signaling cascade. These findings are consistent with observed phenotypes upon inactivation of Sec14p functional homologues in other cell types including mammalian and plant cells, and they delineate a Sec14p-responsive pathway for the regulation of cytokinesis/cell cycle progression.
Acknowledgments
This work was supported by operating grants from the Canadian Institutes of Health Research to C.R.M. and from the National Institutes for Health Research (GM44530) to V.A.B. C.R.M. is supported in part by a Canada Research Chair and A.G.H. by a Nova Scotia Health Research Foundation Graduate Studentship.
Footnotes
Published ahead of print on 29 June 2007.
REFERENCES
- 1.Adamo, J. E., J. J. Moskow, A. S. Gladfelter, D. Viterbo, D. J. Lew, and P. J. Brennwald. 2001. Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J. Cell Biol. 155:581-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aravind, L., A. F. Neuwald, and C. P. Ponting. 1999. Sec14p-like domains in NF1 and Dbl-like proteins indicate lipid regulation of Ras and Rho signaling. Curr. Biol. 9:R195-R197. [DOI] [PubMed] [Google Scholar]
- 3.Bankaitis, V. A., J. R. Aitken, A. E. Cleves, and W. Dowhan. 1990. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature 347:561-562. [DOI] [PubMed] [Google Scholar]
- 4.Bankaitis, V. A., D. E. Malehorn, S. D. Emr, and R. Greene. 1989. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J. Cell Biol. 108:1271-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bankaitis, V. A., S. Phillips, L. Yanagisawa, X. Li, S. Routt, and Z. Xie. 2005. Phosphatidylinositol transfer protein function in the yeast Saccharomyces cerevisiae. Adv. Enzyme Regul. 45:155-170. [DOI] [PubMed] [Google Scholar]
- 6.Bomar, J. M., P. J. Benke, E. L. Slattery, R. Puttagunta, L. P. Taylor, E. Seong, A. Nystuen, W. Chen, R. L. Albin, P. D. Patel, R. A. Kittles, V. C. Sheffield, and M. Burmeister. 2003. Mutations in a novel gene encoding a CRAL-TRIO domain cause human Cayman ataxia and ataxia/dystonia in the jittery mouse. Nat. Genet. 35:264-269. [DOI] [PubMed] [Google Scholar]
- 7.Bose, I., J. E. Irazoqui, J. J. Moskow, E. S. Bardes, T. R. Zyla, and D. J. Lew. 2001. Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle-regulated phosphorylation of Cdc24p. J. Biol. Chem. 276:7176-7186. [DOI] [PubMed] [Google Scholar]
- 8.Caviston, J. P., S. E. Tcheperegine, and E. Bi. 2002. Singularity in budding: a role for the evolutionarily conserved small GTPase Cdc42p. Proc. Natl. Acad. Sci. USA 99:12185-12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, F., and M. Peter. 2003. Yeasts make their mark. Nat. Cell Biol. 5:294-299. [DOI] [PubMed] [Google Scholar]
- 10.Cleves, A. E., T. P. McGee, E. A. Whitters, K. M. Champion, J. R. Aitken, W. Dowhan, M. Goebl, and V. A. Bankaitis. 1991. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell 64:789-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cvrcková, F., C. De Virgilio, E. Manser, J. R. Pringle, and K. Nasmyth. 1995. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 9:1817-1830. [DOI] [PubMed] [Google Scholar]
- 12.Davis, C. R., T. J. Richman, S. B. Deliduka, J. O. Blaisdell, C. C. Collins, and D. I. Johnson. 1998. Analysis of the mechanisms of action of the Saccharomyces cerevisiae dominant lethal cdc42G12V and dominant negative cdc42D118A mutations. J. Biol. Chem. 273:849-858. [DOI] [PubMed] [Google Scholar]
- 13.Debreceni, B., Y. Gao, F. Guo, K. Zhu, B. Jia, and Y. Zheng. 2004. Mechanisms of guanine nucleotide exchange and Rac-mediated signaling revealed by a dominant negative trio mutant. J. Biol. Chem. 279:3777-3786. [DOI] [PubMed] [Google Scholar]
- 14.Drees, B. L., B. Sundin, E. Brazeau, J. P. Caviston, G. C. Chen, W. Guo, K. G. Kozminski, M. W. Lau, J. J. Moskow, A. Tong, L. R. Schenkman, A. McKenzie III, P. Brennwald, M. Longtine, E. Bi, C. Chan, P. Novick, C. Boone, J. R. Pringle, T. N. Davis, S. Fields, and D. G. Drubin. 2001. A protein interaction map for cell polarity development. J. Cell Biol. 154:549-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eby, J. J., S. P. Holly, F. van Drogen, A. V. Grishin, M. Peter, D. G. Drubin, and K. J. Blumer. 1998. Actin cytoskeleton organization regulated by the PAK family of protein kinases. Curr. Biol. 8:967-970. [DOI] [PubMed] [Google Scholar]
- 16.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420:629-635. [DOI] [PubMed] [Google Scholar]
- 17.Fang, M., B. G. Kearns, A. Gedvilaite, S. Kagiwada, M. Kearns, M. K. Fung, and V. A. Bankaitis. 1996. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J. 15:6447-6459. [PMC free article] [PubMed] [Google Scholar]
- 18.Giansanti, M. G., S. Bonaccorsi, R. Kurek, R. M. Farkas, P. Dimitri, M. T. Fuller, and M. Gatti. 2006. The class I PITP giotto is required for Drosophila cytokinesis. Curr. Biol. 16:195-201. [DOI] [PubMed] [Google Scholar]
- 19.Gulli, M. P., M. Jaquenoud, Y. Shimada, G. Niederhauser, P. Wiget, and M. Peter. 2000. Phosphorylation of the Cdc42 exchange factor Cdc24 by the PAK-like kinase Cla4 may regulate polarized growth in yeast. Mol. Cell 6:1155-1167. [DOI] [PubMed] [Google Scholar]
- 20.Hakimi, M. A., D. W. Speicher, and R. Shiekhattar. 2002. The motor protein kinesin-1 links neurofibromin and merlin in a common cellular pathway of neurofibromatosis. J. Biol. Chem. 277:36909-36912. [DOI] [PubMed] [Google Scholar]
- 21.Hama, H., E. A. Schnieders, J. Thorner, J. Y. Takemoto, and D. B. DeWald. 1999. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 274:34294-34300. [DOI] [PubMed] [Google Scholar]
- 22.Henneberry, A. L., T. A. Lagace, N. D. Ridgway, and C. R. McMaster. 2001. Phosphatidylcholine synthesis influences the diacylglycerol homeostasis required for SEC14p-dependent Golgi function and cell growth. Mol. Biol. Cell 12:511-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henneberry, A. L., M. M. Wright, and C. R. McMaster. 2002. The major sites of cellular phospholipid synthesis and molecular determinants of fatty acid and lipid head group specificity. Mol. Biol. Cell 13:3148-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Höfken, T., and E. Schiebel. 2002. A role for cell polarity proteins in mitotic exit. EMBO J. 21:4851-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howe, A. G., V. Zaremberg, and C. R. McMaster. 2002. Cessation of growth to prevent cell death due to inhibition of phosphatidylcholine synthesis is impaired at 37 degrees C in Saccharomyces cerevisiae. J. Biol. Chem. 277:44100-44107. [DOI] [PubMed] [Google Scholar]
- 26.Im, Y. J., S. Raychaudhuri, W. A. Prinz, and J. H. Hurley. 2005. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature 437:154-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irazoqui, J. E., A. S. Gladfelter, and D. J. Lew. 2003. Scaffold-mediated symmetry breaking by Cdc42p. Nat. Cell Biol. 5:1062-1070. [DOI] [PubMed] [Google Scholar]
- 28.Kipnis, P., N. Thomas, R. Ovalle, and P. N. Lipke. 2004. The ER-Golgi v-SNARE Bet1p is required for cross-linking alpha-agglutinin to the cell wall in yeast. Microbiology 150:3219-3228. [DOI] [PubMed] [Google Scholar]
- 29.Kostenko, E. V., G. M. Mahon, L. Cheng, and I. P. Whitehead. 2005. The Sec14 homology domain regulates the cellular distribution and transforming activity of the Rho-specific guanine nucleotide exchange factor Dbs. J. Biol. Chem. 280:2807-2817. [DOI] [PubMed] [Google Scholar]
- 30.Kozminski, K. G., G. Alfaro, S. Dighe, and C. T. Beh. 2006. Homologues of oxysterol-binding proteins affect Cdc42p- and Rho1p-mediated cell polarization in Saccharomyces cerevisiae. Traffic 7:1224-1242. [DOI] [PubMed] [Google Scholar]
- 31.Li, X., M. P. Rivas, M. Fang, J. Marchena, B. Mehrotra, A. Chaudhary, L. Feng, G. D. Prestwich, and V. A. Bankaitis. 2002. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J. Cell Biol. 157:63-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Litvak, V., N. Dahan, S. Ramachandran, H. Sabanay, and S. Lev. 2005. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat. Cell Biol. 7:225-234. [DOI] [PubMed] [Google Scholar]
- 33.Litvak, V., D. Tian, S. Carmon, and S. Lev. 2002. Nir2, a human homolog of Drosophila melanogaster retinal degeneration B protein, is essential for cytokinesis. Mol. Cell. Biol. 22:5064-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longtine, M. S., C. L. Theesfeld, J. N. McMillan, E. Weaver, J. R. Pringle, and D. J. Lew. 2000. Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol. Cell. Biol. 20:4049-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez, M. C., J. M. Nicaud, H. B. Skinner, C. Vergnolle, J. C. Kader, V. A. Bankaitis, and C. Gaillardin. 1994. A phosphatidylinositol/phosphatidylcholine transfer protein is required for differentiation of the dimorphic yeast Yarrowia lipolytica from the yeast to the mycelial form. J. Cell Biol. 125:113-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMaster, C. R., and R. M. Bell. 1994. Phosphatidylcholine biosynthesis in Saccharomyces cerevisiae. Regulatory insights from studies employing null and chimeric sn-1,2-diacylglycerol choline- and ethanolaminephosphotransferases. J. Biol. Chem. 269:28010-28016. [PubMed] [Google Scholar]
- 37.McMaster, C. R., and R. M. Bell. 1994. Phosphatidylcholine biosynthesis via the CDP-choline pathway in Saccharomyces cerevisiae. Multiple mechanisms of regulation. J. Biol. Chem. 269:14776-14783. [PubMed] [Google Scholar]
- 38.Milligan, S. C., J. G. Alb, Jr., R. B. Elagina, V. A. Bankaitis, and D. R. Hyde. 1997. The phosphatidylinositol transfer protein domain of Drosophila retinal degeneration B protein is essential for photoreceptor cell survival and recovery from light stimulation. J. Cell Biol. 139:351-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Min, K. C., R. A. Kovall, and W. A. Hendrickson. 2003. Crystal structure of human alpha-tocopherol transfer protein bound to its ligand: implications for ataxia with vitamin E deficiency. Proc. Natl. Acad. Sci. USA 100:14713-14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monteoliva, L., M. Sanchez, J. Pla, C. Gil, and C. Nombela. 1996. Cloning of Candida albicans SEC14 gene homologue coding for a putative essential function. Yeast 12:1097-1105. [DOI] [PubMed] [Google Scholar]
- 41.Murray, J. P., and C. R. McMaster. 2005. Nte1p-mediated deacylation of phosphatidylcholine functionally interacts with Sec14p. J. Biol. Chem. 280:8544-8552. [DOI] [PubMed] [Google Scholar]
- 42.Nakase, Y., T. Nakamura, A. Hirata, S. M. Routt, H. B. Skinner, V. A. Bankaitis, and C. Shimoda. 2001. The Schizosaccharomyces pombe spo20+ gene encoding a homologue of Saccharomyces cerevisiae Sec14 plays an important role in forespore membrane formation. Mol. Biol. Cell 12:901-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newsome, T. P., S. Schmidt, G. Dietzl, K. Keleman, B. Asling, A. Debant, and B. J. Dickson. 2000. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell 101:283-294. [DOI] [PubMed] [Google Scholar]
- 44.Novick, P., C. Field, and R. Schekman. 1980. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21:205-215. [DOI] [PubMed] [Google Scholar]
- 45.Peter, M., A. M. Neiman, H. O. Park, M. van Lohuizen, and I. Herskowitz. 1996. Functional analysis of the interaction between the small GTP binding protein Cdc42 and the Ste20 protein kinase in yeast. EMBO J. 15:7046-7059. [PMC free article] [PubMed] [Google Scholar]
- 46.Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 113:365-375. [DOI] [PubMed] [Google Scholar]
- 47.Richman, T. J., M. M. Sawyer, and D. I. Johnson. 2002. Saccharomyces cerevisiae Cdc42p localizes to cellular membranes and clusters at sites of polarized growth. Eukaryot. Cell 1:458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivas, M. P., B. G. Kearns, Z. Xie, S. Guo, M. C. Sekar, K. Hosaka, S. Kagiwada, J. D. York, and V. A. Bankaitis. 1999. Pleiotropic alterations in lipid metabolism in yeast sac1 mutants: relationship to “bypass Sec14p” and inositol auxotrophy. Mol. Biol. Cell 10:2235-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rohatgi, R., L. Ma, H. Miki, M. Lopez, T. Kirchhausen, T. Takenawa, and M. W. Kirschner. 1999. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97:221-231. [DOI] [PubMed] [Google Scholar]
- 50.Rossman, K. L., C. J. Der, and J. Sondek. 2005. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6:167-180. [DOI] [PubMed] [Google Scholar]
- 51.Rossman, K. L., D. K. Worthylake, J. T. Snyder, D. P. Siderovski, S. L. Campbell, and J. Sondek. 2002. A crystallographic view of interactions between Dbs and Cdc42: PH domain-assisted guanine nucleotide exchange. EMBO J. 21:1315-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Routt, S. M., and V. A. Bankaitis. 2004. Biological functions of phosphatidylinositol transfer proteins. Biochem. Cell Biol. 82:254-262. [DOI] [PubMed] [Google Scholar]
- 53.Routt, S. M., M. M. Ryan, K. Tyeryar, K. E. Rizzieri, C. Mousley, O. Roumanie, P. J. Brennwald, and V. A. Bankaitis. 2005. Nonclassical PITPs activate PLD via the Stt4p PtdIns-4-kinase and modulate function of late stages of exocytosis in vegetative yeast. Traffic 6:1157-1172. [DOI] [PubMed] [Google Scholar]
- 54.Schekman, R., and P. Novick. 2004. 23 genes, 23 years later. Cell 116:S13-S15. [DOI] [PubMed] [Google Scholar]
- 55.Sha, B., S. E. Phillips, V. A. Bankaitis, and M. Luo. 1998. Crystal structure of the Saccharomyces cerevisiae phosphatidylinositol-transfer protein. Nature 391:506-510. [DOI] [PubMed] [Google Scholar]
- 56.Shang, X., Y. T. Zhou, and B. C. Low. 2003. Concerted regulation of cell dynamics by BNIP-2 and Cdc42GAP homology/Sec14p-like, proline-rich, and GTPase-activating protein domains of a novel Rho GTPase-activating protein, BPGAP1. J. Biol. Chem. 278:45903-45914. [DOI] [PubMed] [Google Scholar]
- 57.Shimada, Y., P. Wiget, M. P. Gulli, E. Bi, and M. Peter. 2004. The nucleotide exchange factor Cdc24p may be regulated by auto-inhibition. EMBO J. 23:1051-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sreenivas, A., J. L. Patton-Vogt, V. Bruno, P. Griac, and S. A. Henry. 1998. A role for phospholipase D (Pld1p) in growth, secretion, and regulation of membrane lipid synthesis in yeast. J. Biol. Chem. 273:16635-16638. [DOI] [PubMed] [Google Scholar]
- 59.Tian, D., V. Litvak, M. Toledo-Rodriguez, S. Carmon, and S. Lev. 2002. Nir2, a novel regulator of cell morphogenesis. Mol. Cell. Biol. 22:2650-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toenjes, K., D. Simpson, and D. I. Johnson. 2004. Separate membrane targeting and anchoring domains function in the localization of the S. cerevisiae Cdc24p guanine nucleotide exchange factor. Curr. Genet. 45:257-264. [DOI] [PubMed] [Google Scholar]
- 61.Ueda, S., T. Kataoka, and T. Satoh. 2004. Role of the Sec14-like domain of Dbl family exchange factors in the regulation of Rho family GTPases in different subcellular sites. Cell. Signal. 16:899-906. [DOI] [PubMed] [Google Scholar]
- 62.Versele, M., and J. Thorner. 2004. Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4. J. Cell Biol. 164:701-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vincent, P., M. Chua, F. Nogue, A. Fairbrother, H. Mekeel, Y. Xu, N. Allen, T. N. Bibikova, S. Gilroy, and V. A. Bankaitis. 2005. A Sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J. Cell Biol. 168:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitehead, I. P., Q. T. Lambert, J. A. Glaven, K. Abe, K. L. Rossman, G. M. Mahon, J. M. Trzaskos, R. Kay, S. L. Campbell, and C. J. Der. 1999. Dependence of Dbl and Dbs transformation on MEK and NF-κB activation. Mol. Cell. Biol. 19:7759-7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wild, A. C., J. W. Yu, M. A. Lemmon, and K. J. Blumer. 2004. The p21-activated protein kinase-related kinase Cla4 is a coincidence detector of signaling by Cdc42 and phosphatidylinositol 4-phosphate. J. Biol. Chem. 279:17101-17110. [DOI] [PubMed] [Google Scholar]
- 66.Wong, T. A., G. D. Fairn, P. P. Poon, M. Shmulevitz, C. R. McMaster, R. A. Singer, and G. C. Johnston. 2005. Membrane metabolism mediated by Sec14 family members influences Arf GTPase activating protein activity for transport from the trans-Golgi. Proc. Natl. Acad. Sci. USA 102:12777-12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie, Z., M. Fang, and V. A. Bankaitis. 2001. Evidence for an intrinsic toxicity of phosphatidylcholine to Sec14p-dependent protein transport from the yeast Golgi complex. Mol. Biol. Cell 12:1117-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie, Z., M. Fang, M. P. Rivas, A. J. Faulkner, P. C. Sternweis, J. A. Engebrecht, and V. A. Bankaitis. 1998. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc. Natl. Acad. Sci. USA 95:12346-12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu, Y., Y. Liu, N. D. Ridgway, and C. R. McMaster. 2001. Novel members of the human oxysterol-binding protein family bind phospholipids and regulate vesicle transport. J. Biol. Chem. 276:18407-18414. [DOI] [PubMed] [Google Scholar]
- 70.Zaremberg, V., C. Gajate, L. M. Cacharro, F. Mollinedo, and C. R. McMaster. 2005. Cytotoxicity of an anti-cancer lysophospholipid through selective modification of lipid raft composition. J. Biol. Chem. 280:38047-38058. [DOI] [PubMed] [Google Scholar]
- 71.Ziman, M., J. M. O'Brien, L. A. Ouellette, W. R. Church, and D. I. Johnson. 1991. Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol. Cell. Biol. 11:3537-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ziman, M., D. Preuss, J. Mulholland, J. M. O'Brien, D. Botstein, and D. I. Johnson. 1993. Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol. Biol. Cell 4:1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]