Abstract
The mechanism of fibroblast-like synoviocyte (FLS) transformation into an inflammatory phenotype in rheumatoid arthritis (RA) is not fully understood. FLS interactions with invading leukocytes, particularly T cells, are thought to be a critical component of this pathological process. Resting T cells and T cells activated through the T-cell receptor have previously been shown to induce inflammatory cytokine production by FLS. More recently, a distinct population of T cells has been identified in RA synovium that phenotypically resembles cytokine-activated T (Tck) cells. Using time lapse microscopy, the interactions of resting, superantigen-activated, and cytokine-activated T cells with FLS were visualized. Rapid and robust adhesion of Tck and superantigen-activated T cells to FLS was observed that resulted in flattening of the T cells and a crawling movement on the FLS surface. Tck also readily activated FLS to produce interleukin IL-6 and IL-8 in a cell contact-dependent manner that was enhanced by exogenous IL-17. Although LFA-1 and ICAM-1 co-localized at the Tck-FLS synapse, blocking the LFA-1/ICAM-1 interaction did not substantially inhibit Tck effector function. However, antibody blocking of membrane tumor necrosis factor (TNF)-α on the Tck surface did inhibit FLS cytokine production, thus illustrating a novel mechanism for involvement of TNF-α in cell-cell interactions in RA synovium and for the effectiveness of TNF-α blockade in the treatment of RA.
The normal synovial lining is one or two cell layers thick, contains both synovial macrophages and fibroblast-like synoviocytes (FLS), and is responsible for maintaining synovial fluid homeostasis. In rheumatoid arthritis (RA), the synovial lining transforms into the synovial pannus, a multicellular tissue composed of hyperplastic FLS closely intertwined with invading lymphocytes and antigen-presenting cells. The current consensus is that FLS are not merely bystanders in the inflammatory process but are active participants in joint destruction.1,2,3,4,5 However, the mechanisms underlying this inflammatory transformation are not yet well understood.
The most prominent invading lymphocyte in the RA synovium is the T cell.6,7,8 T-cell interactions with professional antigen-presenting cells have been well characterized, but in vitro studies have also shown that T cells can adhere to and activate FLS. Resting, unstimulated T cells have been shown to alter gene expression in FLS and cause release of interleukin IL-6, IL-8, and prostaglandin E2,9 and this is augmented by the T-cell cytokine IL-17,9 which is present in RA synovium.10 This effector function of resting T cells was not confined to a unique T-cell subset, as CD4+, CD8+, CD45RA+, and CD45RO+ T cells all had a similar capacity to activate FLS. T cells recovered from RA synovial fluid likewise elicited strong inflammatory responses when cultured with FLS, in particular up-regulating the immune-modulating cytokine IL-15.11 As a prototype for autoreactive T cells that are potentially arthritogenic, collagen II-stimulated T cells enhanced FLS production of many inflammatory mediators, and this effector function appeared to be proportional to the length of T-cell activation with collagen.12
A distinct T-cell activation state has been characterized for its ability to induce tumor necrosis factor TNF-α production from monocytes. These cytokine-activated T cells (Tck) were activated in vitro by a cocktail of IL-6, IL-2, and TNF-α, without T-cell antigen receptor (TCR) ligation. Similarities of function and surface markers have been established between Tck cells and a population of T cells that are abundant in RA synovia.13 However, interactions of Tck cells with FLS have not yet been described.
Conversely, FLS have also been shown to positively stimulate T cells. Simple co-culture of T cells with FLS enhances T-cell survival and prevents apoptosis.14,15 More robust responses have also been reported. FLS can induce naive T-cell proliferation by presentation of superantigen.16,17 When co-cultured with FLS, T cells up-regulate the activation markers CD69 and CD25,11,18 as well as many cytokines, such as IL-17, TNF-α, and interferon-γ.11,12 FLS can also present peptides derived from type II collagen and human cartilage glycoprotein 39 to T-cell hybridomas specific to those peptides.19 These antigen-dependent interactions of FLS with T cells are contact-dependent and can be blocked by neutralizing antibodies to class II major histocompatibility complex19 and LFA-1 or ICAM-1.16
The current studies were undertaken to better define the molecular basis of T-cell/FLS functional interactions. We first sought to directly record in vitro interactions of resting, superantigen-activated, and Tck T cells with FLS by time lapse microscopy. Both Tck and superantigen-activated T cells formed tight associations with FLS that followed similar morphology and kinetics. By further characterizing the interaction of FLS with Tck, it was found that Tck were able to stimulate FLS to produce proinflammatory cytokines in a contact-dependent manner similar to previous observations with resting and antigen-stimulated T cells. However, unlike other T-cell/FLS interactions that were blocked with antibodies to LFA-1/ICAM-1, Tck cell interactions with FLS were not substantially inhibited by antibodies to LFA-1 or ICAM-1 but were in part dependent on surface TNF-α expression by Tck.
Materials and Methods
FLS Isolation and Culture
All procedures involving specimens obtained from human patients were performed under protocols approved by the University of Michigan Institutional Review Board. FLS were obtained from human synovial tissue obtained at arthroplasty or synovectomy from RA joints. RA diagnosis was based on the presence of at least four of the seven American College of Rheumatology criteria for RA.20 FLS were isolated by digestion of synovial tissue with 1 mg/ml of type II collagenase (Worthington Biochemical, Lakewood, NJ) for 2 to 24 hours followed by adherence in cell culture flasks and vigorous washes to remove debris and nonadherent cells.21 Adherent cells were grown to confluency in CMRL medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (Atlanta Biological, Lawrenceville, GA), 2 mmol/L glutamine (Cambrex, East Rutherford, NJ), 50 U/ml penicillin (Cambrex), and 50 μg/ml streptomycin (Cambrex) and then passaged into fresh flasks. RA FLS used before passage 4 were depleted of CD14-positive cells at passage 1 by magnetic bead separation (Miltenyi Biotech, Auburn, CA) to remove contaminating monocyte/macrophage lineage cells.
T-Cell Generation
T cells were obtained from peripheral blood by negative selection using RosetteSep, human T-cell enrichment cocktail (Stemcell Technologies, Vancouver, BC, Canada) and separation on a Ficoll gradient following the manufacturer’s protocol. In brief, freshly isolated blood from healthy volunteers was incubated with antibodies for 20 minutes at room temperature. Blood was diluted 1:1 with 2% newborn calf serum/phosphate-buffered saline (PBS) before overlay onto room temperature Ficoll and centrifugation at 1200 relative centrifugal force with the brake off. The buffy coat containing only T cells was recovered and used for subsequent experiments. Trest were unstimulated T cells. Tck were cytokine-activated T cells stimulated by a cocktail of 25 ng/ml TNF-α, 100 ng/ml IL-6, and 25 ng/ml IL-2 in RPMI 1640 medium containing 10% fetal calf serum, 2 mmol/L l-glutamine, penicillin, and streptomycin for 8 days13 and washed three times before use. Tsea were T cells co-cultured with FLS in the presence of 40 ng/ml of the superantigen Staphylococcal enterotoxin A.16
Tck and FLS Co-Culture for Cytokine Production
FLS were seeded into 12-well plates at a density of 10,000 cells/well and allowed to adhere for 48 hours before the addition of 250,000 cytokine-activated (Tck) cells. Where indicated, recombinant human IL-17 (R&D Systems, Minneapolis, MN) was added to co-cultures to yield a final concentration of 20 ng/ml. Final culture volumes were 1.5 ml/well. Co-culture times were between 24 and 72 hours depending on the assay. Co-culture supernatants were harvested and assayed by enzyme-linked immunosorbent assay (ELISA).
ELISA
ELISAs for IL-6 and IL-8 were performed following the manufacturer’s protocols (BD Biosciences, San Jose, CA). Capture antibodies were coated on enzyme immunoassay plates at a 1:250 dilution in carbonate buffer (pH 9.5) overnight at 4°C. Plates were washed and blocked with PBS:10% fetal calf serum for 1 hour at room temperature before the addition of culture supernatants and recombinant standards followed by reincubation at room temperature. After 2 hours, culture supernatant was washed away and 1:250 dilutions of biotinylated detection antibodies and streptavidin-horseradish peroxidase were added for 1 hour at room temperature. Plates were then washed seven times, 0.1 ml of a 1:1 mixture of TMB substrate was added to each well, and substrate conversion was terminated by addition of 0.1 ml of 2 N H2SO4. Absorbance was read at 450 nm, and sample values were compared with the standard curves.
Time Lapse Microscopy
RA FLS (10,000/well) were plated on 24-well ImageLock plates (Essen Instruments, Ann Arbor, MI) and cultured in CMRL media containing 10% fetal bovine serum and antibiotics in an atmosphere of 5% CO2 for 2 days. Culture medium was removed, and FLS were washed twice with serum-free RPMI 1640 medium before the addition of 250,000 prewarmed, 5(6)-carboxyfluorescein diacetate N-succinimidyl ester-labeled, T cells in RPMI 1640 medium containing 10% fetal calf serum, 2 mmol/L l-glutamine, penicillin, and streptomycin. Plates containing T cells and FLS were immediately placed into IncuCyte-FLR imaging system (Essen Instruments) that is equipped with an LED for excitation at a nominal center wavelength of 470 nm and a FWHM bandwidth of 25 nm. This source is filtered with a short-pass filter with a cutoff wavelength of 490 nm, which ensures that there are no tails of the LED illumination that would get through the emission filter. The detection filter used is a bandpass filter with a 75-nm width centered at 545 nm. The chamber is designed to fit into a standard, humidified, CO2 incubator, and a moving objective allows the cell culture to be stationary while images are captured at different positions from well to well. Images (1-μm resolution) were collected at 10-minute intervals starting 30 minutes after addition of the plate to the IncuCyte-FLR chamber. Movies were generated using IncuCyte software (Essen) at three frames/second, which is equivalent to 30 minutes of culture/second. Still images were cropped and expanded to four times the size of the original images and then exported to Microsoft PowerPoint (Microsoft, Redmond, WA).
Confocal Microscopy
For co-culture studies comparing CD11a and ICAM-1 localization, FLS were transfected with an expression vector for a fusion protein of ICAM-1 and eGFP. This expression vector was termed CD54-eGFP and was created as follows. Primers were constructed against the sequence described by accession BC015969 to polymerase chain reaction (PCR) amplify the open reading frame (without the stop codon) of human ICAM-1. A HindIII restriction site added to the beginning of 5′ primer and an AgeI site added to the tail of the 3′ primer facilitated ligation of the amplified fragment into the pEGFP-N1 expression vector (Clontech, Palo Alto, CA). After ligation, the insert sequence was verified to be free of mutation. This resulted in a CD54-eGFP fusion protein expression vector, with ICAM-1 at the N terminus and eGFP at the C terminus (intracellular) and a short peptide linker (GSTPVAT) in between. Transfection was performed using an adult human dermal fibroblast kit (Amaxa, Gaithersburg, MD), and manufacturer’s protocols were observed. FLS (20,000 to 50,000 cells) were grown on glass coverslips in six-well plates for 48 hours. Medium was changed, and 500,000 to 1 million Tck cells were added to the cultures. Co-cultures were allowed to interact for 1 to 24 hours before coverslips were washed with PBS and fixed in 4% paraformaldehyde/PBS for 1 hour at room temperature or overnight at 4°C. After fixation, coverslips were washed in 2% newborn calf serum/PBS to remove residual paraformaldehyde and blocked in 5% nonfat milk/PBS for 30 minutes. Coverslips were again washed with 2% newborn calf serum/PBS. Primary antibody used for staining was mouse anti-human CD11a (clone HB202; a kind gift of Bruce Richardson, University of Michigan, Ann Arbor, MI).16 Secondary antibody used was goat anti-mouse IgG Alexa 594 (Invitrogen-Molecular Probes, Carlsbad, CA). Confocal imaging was performed using an FV-500 microscope (Olympus, Center Valley, PA) with excitation by argon 488 nm and HeNeG 543 lasers (×60 magnification, 1.4 NA, UPLAN APO).
Transwell Assays
FLS were seeded into 12-well plates at a density of 10,000 per well and allowed to adhere for 48 hours to the bottom chamber. Transwell inserts with a 0.4-μm membrane pore size were placed on top of the wells containing FLS. These transwell inserts had been placed in medium for 24 hours before the addition to plates containing FLS, to saturate the membrane. Tck (n = 500,000) were added to the bottom chamber or into the transwell insert. Total volume in the bottom chamber was 1.5 ml. Total volume in the transwell was 0.5 ml. IL-17 was added to the transwell insert at a 0.04, 0.4, and 4 ng/ml concentration in 0.5 ml but would diffuse through the transwell to give a final concentration of 0.01, 0.1, and 1.0 ng/ml, respectively.
Blocking Studies
FLS were seeded into 12-well plates at a density of 10,000 cells/well and allowed to adhere for 48 hours. Antibodies used for blocking studies were anti-huCD11a (anti-LFA-1, clone HB202; B. Richardson, University of Michigan), anti-huCD49d (anti-VLA-4, clone BU49; Ancell, Bayport, MN), anti-huTNF-α (clones 28401 or 6401; R&D Systems), or control IgG from normal mouse serum (Hybridoma Core, University of Michigan). Tck were incubated at room temperature with 10 μg/ml of blocking antibodies for 2 hours and then washed before addition of 250,000 Tck cells to washed adherent FLS lines. Supernatants were collected after 72 hours of co-culture and tested for IL-6 and IL-8 by ELISA. In some experiments, one of two TNF-α blocking antibodies from different clones (28401 or 6401; R&D Systems) or control mouse IgG were used to coat Tck at 1 μg/ml for 30 minutes at room temperature. Tck cells (250,000 cells/well) were then added to FLS-containing wells with additional antibody added to the co-culture to maintain a final antibody concentration of 1 μg/ml. IL-17 was titrated into these co-cultures in the following concentrations: 0.02, 0.2, and 2.0 ng/ml (we found similar results using 2 and 20 ng/ml IL-17, data not shown). Co-cultures were then maintained for 24 to 72 hours before collection of supernatants. For studies that examined blocking of surface TNF-α, Tck were incubated for 30 minutes with control or TNF-α-specific antibodies. Excess unbound antibody was removed by washes with 2% newborn calf serum in PBS. These preblocked Tck were used to stimulate FLS. Alternatively, anti-TNF-α or control MsIg antibodies were added directly to the co-cultures of Tck and RA FLS at a final concentration of 1 μg/ml. Supernatants were harvested after 36 hours of co-culture and IL-8 was detected by ELISA.
RT-PCR
Trest and Tck were obtained from the same donor. U937 cells were treated with phorbol 12-myristate 13-acetate (2 μg/ml) for 2 hours before harvest. RNA was extracted from 5 million cells of each cell type using the RNeasy kit (Qiagen, Valencia, CA). Reverse transcription was performed on 2 μg of RNA from each cell type for 50 minutes at 37°C. A second reverse transcription was performed after treating the RNA with 1 U of RNase to exclude the possibility of amplifying contaminating genomic DNA. The resultant cDNA products were diluted 2:3 before use in PCR. One μl of the diluted cDNA was used for each PCR reaction. TNF-α primer sequences were 5′-CAGAGGGAAGAGTTCCCCAG-3′ for the upstream primer and 5′-CCTTGGTCTGGTAGGAGACG-3′ for the downstream primer.22 β-Actin served as the control. The β-actin primers were 5′-GTGGGGCGCCCCAGGCACCA-3′ for the upstream primer and 5′-CTCCTTAATGTCACGCACGATTTC-3′ for the downstream primer. PCR amplification was performed in a PX2 thermocycler (Thermo Hybaid, Franklin, MA) as follows: first step, one cycle of 94°C for 3 minutes; second step, 30 cycles (1 minute at 94°C, 1 minute at 60°C, 40 seconds at 72°C); and third step, one cycle extension for 5 minutes at 72°C. PCR products were analyzed by electrophoresis on a 2% agarose gel containing 1× SYBR Safe DNA gel stain (Invitrogen), and band size was compared with a 100-bp molecular weight marker (Promega, Madison, WI).
Results
Cytokine-Activated T Cells Adhere Tightly to FLS in Co-Cultures
Previous work in our laboratory used two types of T cells, Trest and Tsea,9,16 which could be viewed as representing polar states of T-cell stimulation from naïve to full activation. An alternative of T-cell activation has been described, termed cytokine-activated T cells (Tck). Tck are generated by stimulation independent of the TCR by a cocktail of cytokines: TNF-α, IL-6, and IL-2. These cytokines, especially TNF-α and IL-6, are found within synovial tissues. Tck have been documented to possess effector function identical to T cells purified from RA synovial fluid, most notably in the induction of TNF production by monocytes. The stimulatory mechanism of these cells is clearly distinct from T cells activated through CD3/CD28 cross-linking.13 We therefore sought to explore Tck interactions with FLS.
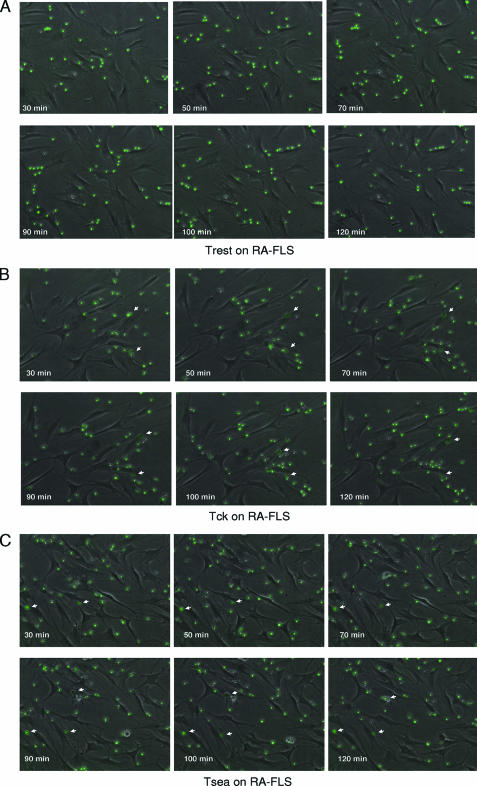
The method of generation of Tck yielded a nearly homogeneous population of 94% CD3-positive T cells with little or no contamination by B cells, monocytes, or fibroblasts (see Supplemental Table 1 at http://ajp.amjpathol.org). To observe the physical interactions between FLS and differentially activated T cells, co-cultures were filmed using time lapse microscopy (see Supplemental Video S1A–C at http:// ajp.amjpathol.org). Three activation states of T cells were used, unstimulated (Trest), Staphylococcal enterotoxin A-stimulated (Tsea), and cytokine-activated T cells (Tck). Representative images from time lapse microscopy of the co-cultures show significant cellular interaction of the 5(6)-carboxyfluorescein diacetate N-succinimidyl ester-labeled Tck and Tsea but not Trest cells with FLS within the first 30 minutes of co-culture (Figure 1, A–C). When the activated T cells adhered to FLS, they acquired an enlarged, darker appearance (indicated by white arrows) than T cells with only minimal interactions. In contrast, Trest cells maintained a smaller, brighter appearance despite being proximal to FLS, and unlike their activated counterparts, did not appear to crawl over the surface of the FLS. Further evidence of a difference in interactions between Trest and activated T cells can be witnessed by following the movement of individual cells in relation to the movement of the FLS (see Supplemental Video S1A–C at http://ajp.amjpathol.org). When FLS moved in the presence of Trest, the T cells tended to break contact with the FLS and move to another position in the well. In contrast, Tck and Tsea tended to stay attached to one FLS throughout the 24-hour assay period despite the movement of the FLS. No significant differences between the interactions of Tsea or Tck with FLS were observed using time lapse microscopy.
Figure 1.
Tsea and Tck adhere tightly to RA FLS. Still images from time lapse microscopy of resting T cells (Trest) (A), cytokine-activated T cells (Tck) (B), and Staphylococcal enterotoxin A-stimulated T cells (Tsea) (C) co-cultured with FLS from RA patients (RA-FLS). 5(6)-Carboxyfluorescein diacetate N-succinimidyl ester-labeled T cells (250,000/well) were added to adherent RA-FLS and images were captured from 0.5 to 24 hours at 10-minute intervals (full movies attached as Supplemental Video S1A–C at http://ajp.amjpathol.org). Time stamp shows the time from the beginning of the co-culture. White arrows mark examples of Tck and Tsea cells that are tightly adherent to the surface of RA-FLS. Such tightly adherent cells are found much less frequently in the Trest co-culture on RA-FLS.
Cytokine-Activated T Cells Induce FLS Production of Inflammatory Cytokines
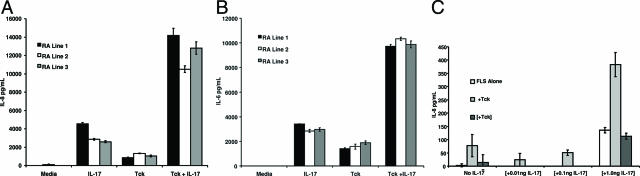
FLS cultures were stimulated by the addition of Tck and allowed to interact for 36 hours. The cytokine IL-17, produced by RA synovial tissues,10 has been shown to cooperate with Trest in the activation of FLS9 and was also included in some wells. Three RA FLS lines were assayed for stimulation by Tck (Figure 2, A and B). Tck stimulated FLS to produce the inflammatory cytokines IL-6 and IL-8, and this effector function cooperated significantly with IL-17. No IL-6 or IL-8 production was detected in cultures of Tck and IL-17 in the absence of FLS (data not shown). OA FLS lines behaved similarly to RA FLS in regards to IL-6 and IL-8 production induced by stimulation with Tck (data not shown).
Figure 2.
Tck activate FLS in a cell contact-dependent manner. Tck were co-cultured with three different RA-FLS lines. After 36 hours of co-culture, supernatants were harvested, and the resultant IL-8 (A) and IL-6 (B) was measured by ELISA. Bars represent mean values of one experiment representative of two different experiments performed in triplicate. Error bars represent the 95% confidence interval. C: FLS were grown in the bottom chamber of a transwell plate. Tck were added directly into the bottom chamber with FLS (+Tck) or separated from FLS by transwell inserts ([+Tck]). Control conditions were FLS alone. IL-17 was titrated into the transwell inserts and allowed to diffuse throughout the co-culture chambers. The values of IL-17 given on the x axis denote the final concentration of IL-17 in the entire culture. The resultant IL-8 production in supernatants was measured by ELISA. Brackets denote additions inside the transwell and not directly to FLS. Bars represent means of one experiment representative of two different experiments performed in triplicate. Error bars represent the 95% confidence interval.
Tck Activation of FLS Requires Cell-Cell Contact
To assess the importance of cell-cell contact in activation of FLS by T cells, transwell inserts were used in co-cultures. Tck were either allowed to interact directly or Tck were separated from FLS by a transwell insert. The insert allowed the free flow of cytokines and other molecules but prevented intercellular adhesive interactions from occurring. IL-17 was added to some of the cultures directly into the upper chamber and allowed to diffuse through the entire culture. In these experiments, IL-8 production and cooperation with IL-17 were observed only when Tck were allowed to directly contact the FLS (Figure 2C). When Tck were separated from FLS by the transwell insert, IL-8 production was similar to IL-17 stimulation, best observed in the 1-ng/ml IL-17 column. This suggested that IL-17 was not acting on Tck to induce production of soluble inflammatory mediators.
LFA-1 Localizes to the Contact Point between FLS and Tck
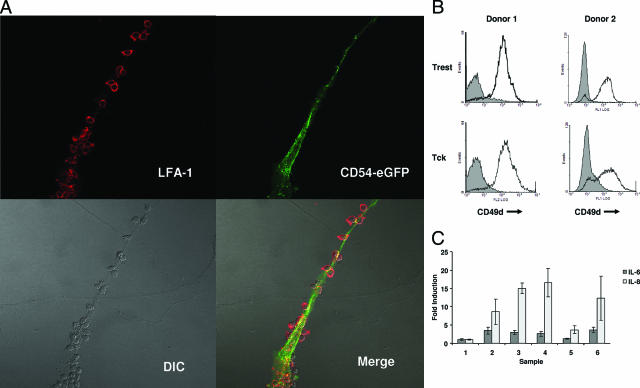
Given the importance of cell-cell contact to Tck effector function toward FLS, we sought to better define the contact point between the two cells. There is evidence that the FLS-adherent population of T cells expresses high levels of the integrin LFA-1.23 It has also been observed that adhesion of T cells to FLS in static assays could be inhibited with blocking antibodies against LFA-1 (CD11a/CD18) and its counter-receptor ICAM-1/CD54.24,25 Beyond adhesion, LFA-1 has been implicated in supplying a co-stimulatory signal to T cells.26 The adhesive and/or co-stimulatory properties of LFA-1 are important in the presentation of superantigens to T cells,27,28,29 and in particular superantigen presentation by FLS.16 ICAM-1 (CD54) is an adhesion molecule that is one of the ligands of LFA-1 and that is expressed abundantly by FLS.30 In addition to adhesion, ICAM-1 ligation induces stimulatory signals in cells that express this molecule.29,31,32,33 We therefore sought to define the contact point, or synapse, between FLS and T cells and determine whether LFA-1 and ICAM-1 co-localized in this region. To observe the localization of ICAM-1 in antigen-independent activation of FLS by Tck, FLS were transfected with a CD54-eGFP expression vector before co-culture with Tck. Co-cultured cells were fixed, and Tck were indirectly stained for CD11a, which was visualized by means of the fluorochrome Alexa 594. At the synapse of Tck and FLS, confocal imaging revealed merged signals between ICAM-1 and LFA-1 (Figure 3A).
Figure 3.
Dependence of Tck interactions with RA-FLS on cell adhesion molecules and TNF-α. A: Confocal imaging of stained co-cultured cells was performed to examine cell-cell interactions. FLS were transfected with an expression vector for CD54-eGFP fusion protein and allowed to adhere to glass coverslips before being co-cultured with Tck. Co-localization of CD11a on Tck surfaces (stained red) and CD54-eGFP on RA-FLS (green) was detected as yellow signal on merged images. DIC, differential interference contrast. B: Flow cytometric analysis of the expression of CD49d on Trest and Tck cells of two healthy individuals. Mean channel fluorescence values were: donor 1: Trest MCF = 11.2 and Tck MCF = 17.7; and donor 2: Trest MCF = 11.9 and Tck MCF = 16.3. C: Tck cells were preincubated with control MsIg or neutralizing antibodies to CD11a (LFA-1), CD49d (VLA-4), or TNF-α before co-culture with RA-FLS. Supernatants were collected at 72 hours of co-culture, and IL-6 and IL-8 levels were measured by ELISA. Samples: 1, no Tck; 2, Tck + control Ab; 3, Tck + anti-LFA-1; 4, Tck + anti-VLA-4; 5, Tck + anti-TNF-α; 6, Tck + anti-LFA-1 + anti-VLA-4. Values are expressed as mean fold induction ± SD compared with cytokines produced by RA-FLS alone. Original magnifications, ×60.
Given the strong co-localization of ICAM-1 and LFA-1 to the contact point of Tck and FLS, we attempted to disrupt the effector function of Tck on FLS using blocking antibodies to these adhesive proteins. Despite co-localization of these structures, blocking antibodies directed against LFA-1 and ICAM-1 did not inhibit Tck stimulation of FLS cytokine production (Figure 3C). Another adhesion molecule found abundantly on Tck cells was VLA-4, detected by staining with anti-CD49d (Figure 3B) that could interact with VCAM-1 expressed by FLS. Similar to the results using anti-LFA-1 alone, blockade of VLA-4 alone or the combination of VLA-4 and LFA-1 adhesion axes had no effect on FLS cytokine production stimulated by Tck cells (Figure 3C).
TNF-α Is Required for Optimal Tck Activation of FLS and Cooperation with IL-17
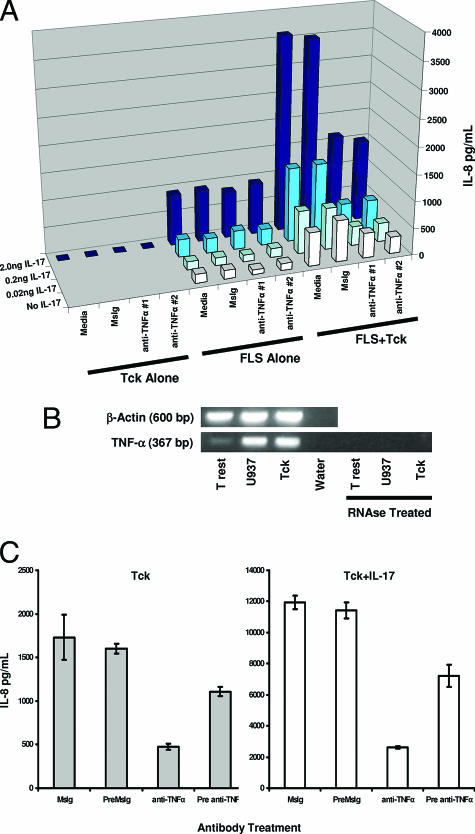
Given the abundance of TNF-α in RA synovial tissue and fluid,34,35 and the use of TNF-α blockade as an effective therapy for RA,36 we also examined the role of TNF-α in Tck/FLS interactions. Although macrophages are considered to be the primary source of TNF-α in RA, T cells are also known to produce TNF-α.37 In contrast to the results for anti-LFA-1 and anti-VLA-4, blockade with neutralizing anti-TNF-α antibodies resulted in reversal of the Tck-stimulated cytokine production (Figure 3C).
To further characterize this effect, two different TNF-α-blocking antibodies were used to inhibit Tck/FLS interaction and Tck cooperation with IL-17. TNF-α blockade substantially inhibited the cooperative effect of Tck with IL-17 in activation of FLS as assayed by IL-8 production (Figure 4A). Neither of the TNF-α-blocking antibodies significantly inhibited the effect of IL-17 on FLS in the absence of Tck cells.
Figure 4.
Tck activation of FLS requires T-cell membrane-bound TNF-α. A: IL-8 production from three different cell cultures was compared: Tck cultured alone (Tck alone), FLS cultured alone (FLS alone), or FLS cultured with Tck (FLS + Tck). Before co-culture with FLS, Tck were incubated with either one of two different TNF-α blocking antibodies (anti-TNF-α no. 1 clone 6401 or anti-TNF-α no. 2 clone 28401), control antibody (MsIg), or no antibody (media). The resultant IL-8 production from different conditions is represented by a three-dimensional chart. The x axis displays culture conditions with blocking antibodies used. The y axis shows IL-8 production. The z axis shows the concentration of IL-17 titrated into the cultures and is also represented by increasing color density of blue, ranging from white for no IL-17 to navy blue for 2.0 ng/ml IL-17. Error bars were omitted for clarity but mean values and statistical data are supplied as supplemental data (see Supplemental Table 2 at http://ajp.amjpathol.org). B: RNA was prepared from Trest, Tck, and U937 cells. U937 cells were stimulated with phorbol 12-myristate 13-acetate for 2 hours before being harvested for RNA. cDNA was transcribed from the RNA and analyzed for TNF-α gene expression by PCR amplification. As a control, a portion of the RNA was treated with RNase before the generation of cDNA. β-Actin was also amplified as an internal control. C: Tck were incubated with 1 μg/ml TNF-α-blocking antibody (Pre anti-TNF) or control antibody (PreMsIg) for 30 minutes to bind all surface TNF-α. Excess blocking antibody was removed by sequential washes of Tck with PBS before co-culture with RA-FLS. Alternatively, MsIg and anti-TNF were added directly to the co-cultures of Tck with RA-FLS. IL-17 was added at a final concentration of 2 ng/ml to some culture wells (right), supernatants were collected at 36 hours, and IL-8 was measured by ELISA. Bars represent mean values from one experiment representative of two different experiments performed in triplicate. Error bars represent the 95% confidence interval.
Based on these findings, the TNF-α concentrations in co-culture supernatants were evaluated by ELISA. No secreted TNF-α was detected in any of these cultures (data not shown). However, TNF-α gene activation could be detected in Tck by RT-PCR (Figure 4B). Because transwell assays showed that the dependence of RA FLS activations by Tck was cell-cell contact-dependent (Figure 2C), and TNF-α is known to be expressed as a membrane-bound protein, membrane-bound TNF-α on T cells could be responsible for TNF-α effector function in this experimental system. To assess this possibility, Tck were preincubated with blocking antibody against TNF-α for 30 minutes and washed to remove excess unbound antibody. These antibody-coated Tck were used to activate FLS. Preincubation of Tck with blocking antibody to TNF-α (pre anti-TNF) significantly reduced Tck activation of FLS and cooperation with IL-17 (Figure 4C), although not to the same extent as when excess antibody was present in co-cultures (anti-TNF). This suggested that the membrane-bound TNF was dynamically restored on Tck surfaces once blocking antibody was removed from the culture medium.
Discussion
Accumulating evidence indicates that bidirectional signaling between T cells and FLS is robust and functionally significant.9,16,19 Previous reports have suggested that a large number of T cells found in RA synovium are functionally distinct from resting or antigen-activated T cells and instead resemble T cells that have been activated solely through exposure to the cytokines IL-6, IL-2, and TNF-α (Tck).13,38 However, the functional relationship between Tck and RA FLS has not been previously examined. In the current study, we have used a variety of imaging approaches to demonstrate visually the extent of interactions between differentially activated T cells and RA FLS and have used assays of FLS cytokine production to characterize the outcome of such interactions.
We observed that superantigen-activated and Tck cells readily adhered to and interacted with FLS in a pattern that was qualitatively distinct from resting T cells. We also found that co-culture of Tck with RA FLS led to functional effects, namely FLS activation resulting in IL-6 and IL-8 production. This effector function of Tck cooperated with the effects of the T-cell-specific cytokine IL-17 and required cell-cell contact.
Early work on T-cell/FLS interaction noted that these cells readily adhered in co-culture.23,24,25,39 The CD2/LFA-339 and LFA-1/ICAM-123,24,25 adhesive interactions have been implicated in T-cell/FLS association. The relative requirements of these protein-protein interactions for T-cell adhesion to FLS remains ill defined because different studies report differing effects when these interactions were inhibited using blocking antibodies.24,25,39 These differences were most likely attributable to the varying activation states of the T cells used, as well as differing methodologies used to assess adhesion. However, two studies characterizing the phenotype of adherent T cells and FLS reported increased expression of LFA-1 and ICAM-1, respectively, on these cells.23,25 In addition, cell-cell interactions in T-cell-FLS co-cultures can lead to FLS up-regulation of VCAM-1 and ICAM-1.40
The signaling mechanisms between T cells and FLS have been observed to involve a variety of mechanisms. The LFA-1/ICAM-1 interaction may play a role in this signaling beyond its role in adhesion. ICAM-129,31,32,33 and LFA-126,27,28,41,42 have also been implicated as co-stimulatory structures that can enhance cellular activation. One study previously reported that interference with this interaction could greatly blunt the outcome of T-cell/FLS interactions.11 We have been able to visualize LFA-1/ICAM-1 interactions between T cells and FLS by confocal microscopy and localize these interactions to the contact point of the two cells. Consistent with studies demonstrating the importance of LFA-1 for adhesion of T cells to FLS,23,24,25 LFA-1 was imaged localizing to the contact point between FLS and T cell, even in systems devoid of exogenous antigens, using Tck that were activated independently of the TCR, in conjunction with the counter-receptor ICAM-1 expressed on the FLS surface. However, in our system, inhibition of LFA-1/ICAM-1 by blocking antibody did not result in inhibition of Tck effector function, despite the requirement that Tck directly contact FLS. This suggests that the contribution of the LFA-1/ICAM-1 interactions to Tck effector function on FLS activation was not significant. It seems that the LFA-1/ICAM-1 axis may be more critical for T-cell responses induced through the T-cell receptor by interaction with antigen/major histocompatibility complex expressed on FLS16 than for FLS responses to signals coming from Tck.
Another important adhesive axis between T cells and FLS is thought to be the interaction of VLA-4 (CD49d/CD29) on T cells with VCAM-1 on FLS.43 Unactivated T cells from healthy donors expressed high surface expression of CD49d that was only modestly increased on activation with Tck-stimulating cytokines. Blockade of VLA-4/VCAM-1 interactions alone or in combination with LFA-1 blockade did not alter the ability of Tck to induce cytokine production from RA FLS. Thus, Tck cells seemed to function on RA FLS in a different manner than resting T cells or antigen-stimulated T cells.
Beyond adhesive interactions, T cells and FLS possess other potential signaling structures. The cytokine IL-15 has been identified as a mediator of T-cell and FLS interactions.11 Engagement of the co-stimulatory receptor CD40 on FLS by CD40L on activated T cells results in enhanced VEGF production.44 The CD47/TSP-1 pathway has been shown to be involved in T-cell adhesion and survival in extended co-cultures with FLS.45 The distinct patterns of T-cell movement over and engagement of FLS by functionally diverse T-cell populations, which were imaged by time lapse microscopy in our experiments, can be presumed to reflect distinct engagement of sets of receptors with their FLS ligands by the different types of T cells. A full understanding of the molecular basis for T-cell/FLS interactions will require many additional experiments that use a variety of approaches to alter expression or function of each molecule that is putatively involved.
The current study extends understanding of T-cell/FLS interactions by focusing on the Tck, which provide a convenient in vitro system to generate T cells that function in a manner similar to RA synovial T cells. Tck have been shown to induce TNF-α production from monocytes through a mechanism distinct from pathways used by T cells stimulated through CD3/CD28 engagement. Tck effector function required nuclear factor-κB activity and was inhibited by phosphatidyl inositol 3-kinase activity.13
Tck proved to be potent activators of FLS, increasing production of IL-6 and IL-8 during co-cultures. Similar to earlier experiments with resting T cells9 and consistent with one of its proposed functions,46 IL-17 cooperated with Tck. We found that a major signaling pathway for this interaction was TNF-α on the surface of Tck. TNF-α is found abundantly within rheumatoid synovial tissues34,35,47 and although there is a sizable soluble pool, the contribution of membrane-bound TNF remains ill defined. The binding of TNF-α to one of its two distinct receptors48,49 results in many biological effects that are relevant to the pathogenesis of RA.50 TNF-α is initially synthesized as a transmembrane protein. Soluble TNF-α is produced by shedding of the membrane-bound form by the membrane-associated metalloproteinase TACE.51 Membrane-bound TNF-α is not simply a precursor of the fully functional cytokine but instead has been reported to function in cytotoxic killing.52,53 It has been suggested that these two forms of TNF-α can mediate distinct biological effects.54,55 TNF-α has been shown to be a potent proinflammatory signal for human FLS.56 Moreover, TNF-α neutralization is an effective therapeutic approach in human RA.57,58
In view of the effective use of TNF-α blockade in RA, and reports of IL-17 cooperation with TNF-α in the inflammatory process, we explored TNF-α neutralization as an approach to inhibit Tck function.46,59 The effects of Tck in combination with IL-17 are also interesting in light of the new T-cell effector archetype Th17.60 These cells are now known to be distinct from Th1 or Th2 and are potent contributors to autoimmune disease.61,62 That IL-17 is detectable within synovial tissues suggests that Th17 effectors also may be present.10 Proinflammatory effects of cells similar to Tck would be enhanced in situ by IL-17 derived from Th17 cells. Anti-TNF-α did indeed prove to be an effective inhibitor of Tck effects on FLS and of cooperation between Tck and IL-17. In these experiments, there was no evidence that IL-17 induced production of TNF-α by FLS because TNF-α-blocking antibody had no effect on IL-17 stimulation of FLS. We also found that, on Tck, TNF-α is membrane bound, as indicated by the lack of FLS activation when Tck were separated from FLS by a transwell insert, the blocking effect on FLS activation seen with precoating of Tck with anti-TNF-α, and the lack of detectable soluble TNF-α in the culture medium. Although TNF-α blockade greatly diminished Tck effector function and cooperation with IL-17, effector function was not completely abrogated. This could be attributable to incomplete neutralization of all TNF-α molecules but is most likely attributable to a diversity of signaling pathways between T cells and FLS that were not entirely disrupted by anti-TNF-α.
Although the role of a T-cell population expressing membrane-bound TNF-α has not been extensively studied in RA pathophysiology, research in Crohn’s disease (CD) has documented the pathological functions of these cells. Similar to the dramatic results in RA, anti-TNF-α therapy has proven to be an effective treatment for CD.63,64 In determining the mechanism of this therapeutic effect, it was found that neutralization of TNF-α resulted in lowered interferon-γ and TNF-α production from CD patient T cells.65,66,67 Further study revealed that therapeutic anti-TNF-α monoclonal antibodies exerted their effects by specifically binding to T-cell surfaces. Antibody binding to T-cell membranes led to caspase-3 activation and apoptosis in cell cultures, with lamina propria T cells showing increased susceptibility compared with peripheral blood T cells.68 This apoptotic effect was further verified by endoscopic tissue biopsy of CD patients receiving anti-TNF-α therapy, which indicated in situ T-cell death.69 In systems that are closer to RA, membrane-bound cytokines on T cells activated by phytohemagglutinin have been observed to convey signals to FLS.70,71 These phytohemagglutinin blasts are likely similar to T cells stimulated through the TCR and are functionally and phenotypically distinct from Tck.13
In our studies, we have used a population of T cells that resemble T cells isolated from RA SF to activate FLS. Similar to T cells from CD, these Tck use membrane-bound TNF-α as a mechanism to convey inflammatory signals. This suggests that, along with soluble TNF-α, TNF-α on T-cell surfaces could provide an important signaling interaction between T cells and other cells in RA synovium. These results provide another mechanism for the utility of TNF-α blockade in the treatment of RA and imply that membrane-bound TNF-α on T cells may be important in the pathogenesis of RA and possibly other inflammatory arthritides.
Supplementary Material
Acknowledgments
We thank Donna Cash for preparation of the manuscript.
Footnotes
Address reprint requests to David A. Fox, M.D., Room 3918 Taubman Center, 1500 E. Medical Center Dr., Ann Arbor, MI 48109-0358. E-mail: dfox@umich.edu.
Supported by the National Institute of Environmental Health Sciences (grant 5R01ES11196-5), the National Institute of Arthritis and Musculoskeletal Skin Diseases (grant R-01AR38477), the University of Michigan Rheumatic Disease Core Center, and the Medical Scientist Training Program.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis D, Kollias G. Fibroblast biology. Synovial fibroblasts in rheumatoid arthritis: leading role or chorus line? Arthritis Res. 2000;2:342–343. doi: 10.1186/ar109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinecke I, Rutkauskaite E, Gay S, Pap T. The role of synovial fibroblasts in mediating joint destruction in rheumatoid arthritis. Curr Pharm Des. 2005;11:563–568. doi: 10.2174/1381612053381945. [DOI] [PubMed] [Google Scholar]
- Mor A, Abramson SB, Pillinger MH. The fibroblast-like synovial cell in rheumatoid arthritis: a key player in inflammation and joint destruction. Clin Immunol. 2005;115:118–128. doi: 10.1016/j.clim.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Müller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, Gay S. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996;149:1607–1615. [PMC free article] [PubMed] [Google Scholar]
- Abrahamsen TG, Froland SS, Natvig JB, Pahle J. Elution and characterization of lymphocytes from rheumatoid inflammatory tissue. Scand J Immunol. 1975;4:823–830. doi: 10.1111/j.1365-3083.1975.tb03723.x. [DOI] [PubMed] [Google Scholar]
- Bankhurst AD, Husby G, Williams RC., Jr Predominance of T cells in the lymphocytic infiltrates of synovial tissues in rheumatoid arthritis. Arthritis Rheum. 1976;19:555–562. doi: 10.1002/art.1780190307. [DOI] [PubMed] [Google Scholar]
- Van Boxel JA, Paget SA. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975;293:517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Yamamura Y, Gupta R, Morita Y, He X, Pai R, Endres J, Freiberg A, Chung K, Fox DA. Effector function of resting T cells: activation of synovial fibroblasts. J Immunol. 2001;166:2270–2275. doi: 10.4049/jimmunol.166.4.2270. [DOI] [PubMed] [Google Scholar]
- Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Miranda-Carús ME, Balsa A, Benito-Miguel M, Perez de Ayala C, Martin-Mola E. IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate. J Immunol. 2004;173:1463–1476. doi: 10.4049/jimmunol.173.2.1463. [DOI] [PubMed] [Google Scholar]
- Kim WU, Cho ML, Jung YO, Min SY, Park SW, Min DJ, Yoon JH, Kim HY. Type II collagen autoimmunity in rheumatoid arthritis. Am J Med Sci. 2004;327:202–211. doi: 10.1097/00000441-200404000-00006. [DOI] [PubMed] [Google Scholar]
- Brennan FM, Hayes AL, Ciesielski CJ, Green P, Foxwell BM, Feldmann M. Evidence that rheumatoid arthritis synovial T cells are similar to cytokine-activated T cells: involvement of phosphatidylinositol 3-kinase and nuclear factor κB pathways in tumor necrosis factor alpha production in rheumatoid arthritis. Arthritis Rheum. 2002;46:31–41. doi: 10.1002/1529-0131(200201)46:1<31::AID-ART10029>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Scott S, Pandolfi F, Kurnick JT. Fibroblasts mediate T cell survival: a proposed mechanism for retention of primed T cells. J Exp Med. 1990;172:1873–1876. doi: 10.1084/jem.172.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon M, Scheel-Toellner D, Huissoon AP, Pilling D, Shamsadeen N, Hyde H, D’Angeac AD, Bacon PA, Emery P, Akbar AN. Inhibition of T cell apoptosis in the rheumatoid synovium. J Clin Invest. 1997;99:439–446. doi: 10.1172/JCI119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C, Diaz LA, Jr, Singer NG, Li LL, Kirsch AH, Mitra R, Nickoloff BJ, Crofford LJ, Fox DA. Responsiveness of human T lymphocytes to bacterial superantigens presented by cultured rheumatoid arthritis synoviocytes. Arthritis Rheum. 1996;39:125–136. doi: 10.1002/art.1780390117. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE, Guyre PM. Increased proliferation of human synovial fibroblasts treated with recombinant immune interferon. J Immunol. 1985;134:3142–3146. [PubMed] [Google Scholar]
- Corrigall VM, Solau-Gervais E, Panayi GS. Lack of CD80 expression by fibroblast-like synoviocytes leading to anergy in T lymphocytes. Arthritis Rheum. 2000;43:1606–1615. doi: 10.1002/1529-0131(200007)43:7<1606::AID-ANR26>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Tran CN, Davis MJ, Tesmer LA, Endres JL, Motyl CD, Smuda C, Somers EC, Chung KC, Urquhart AG, Lundy SK, Kovats S, Fox DA. Presentation of arthritogenic peptide to antigen-specific T cells by fibroblast-like synoviocytes. Arthritis Rheum. 2007;56:1497–1506. doi: 10.1002/art.22573. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Zimmermann T, Kunisch E, Pfeiffer R, Hirth A, Stahl H-D, Sack U, Laube A, Liesaus E, Roth A, Palombo-Kinne E, Emmrich F, Kinne RW. Isolation and characterization of rheumatoid arthritis synovial fibroblasts from primary culture—primary culture cells markedly differ from fourth-passage cells. Arthritis Res. 2001;3:72–76. doi: 10.1186/ar142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber HP, Mikkila A, Erich JM, Kroger N, Meyer A, Schoder V, Zander AR, Pforte A. TNFα, interleukin-10 and interleukin-18 expression in cells of the bronchoalveolar lavage in patients with pulmonary complications following bone marrow or peripheral stem cell transplantation: a preliminary study. Bone Marrow Transplant. 2002;30:485–490. doi: 10.1038/sj.bmt.1703722. [DOI] [PubMed] [Google Scholar]
- Matsuoka N, Eguchi K, Kawakami A, Ida H, Nakashima M, Sakai M, Terada K, Inoue S, Kawabe Y, Kurata A. Phenotypic characteristics of T cells interacted with synovial cells. J Rheumatol. 1991;18:1137–1142. [PubMed] [Google Scholar]
- Krzesicki RF, Fleming WE, Winterrowd GE, Hatfield CA, Sanders ME, Chin JE. T lymphocyte adhesion to human synovial fibroblasts. Role of cytokines and the interaction between intercellular adhesion molecule 1 and CD11a/CD18. Arthritis Rheum. 1991;34:1245–1253. doi: 10.1002/art.1780341007. [DOI] [PubMed] [Google Scholar]
- Nakatsuka K, Tanaka Y, Hubscher S, Abe M, Wake A, Saito K, Morimoto I, Eto S. Rheumatoid synovial fibroblasts are stimulated by the cellular adhesion to T cells through lymphocyte function associated antigen-1/intercellular adhesion molecule-1. J Rheumatol. 1997;24:458–464. [PubMed] [Google Scholar]
- Geppert TD, Lipsky PE. Activation of T lymphocytes by immobilized monoclonal antibodies to CD3. Regulatory influences of monoclonal antibodies to additional T cell surface determinants. J Clin Invest. 1988;81:1497–1505. doi: 10.1172/JCI113481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JM, Zheng XG, Shimizu Y, Thompson CB, Turka LA. T cell receptor stimulation, but not CD28 costimulation, is dependent on LFA-1-mediated events. Eur J Immunol. 1994;24:265–272. doi: 10.1002/eji.1830240141. [DOI] [PubMed] [Google Scholar]
- Fischer H, Gjorloff A, Hedlund G, Hedman H, Lundgren E, Kalland T, Sjogren HO, Dohlsten M. Stimulation of human naive and memory T helper cells with bacterial superantigen. Naive CD4+45RA+ T cells require a costimulatory signal mediated through the LFA-1/ICAM-1 pathway. J Immunol. 1992;148:1993–1998. [PubMed] [Google Scholar]
- Labuda T, Wendt J, Hedlund G, Dohlsten M. ICAM-1 costimulation induces IL-2 but inhibits IL-10 production in superantigen-activated human CD4+ T cells. Immunology. 1998;94:496–502. doi: 10.1046/j.1365-2567.1998.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale LP, Martin ME, McCollum DE, Nunley JA, Springer TA, Singer KH, Haynes BF. Immunohistologic analysis of the distribution of cell adhesion molecules within the inflammatory synovial microenvironment. Arthritis Rheum. 1989;32:22–30. doi: 10.1002/anr.1780320105. [DOI] [PubMed] [Google Scholar]
- Holland J, Owens T. Signaling through intercellular adhesion molecule 1 (ICAM-1) in a B cell lymphoma line. The activation of Lyn tyrosine kinase and the mitogen-activated protein kinase pathway. J Biol Chem. 1997;272:9108–9112. doi: 10.1074/jbc.272.14.9108. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Tanaka Y, Saito K, Abe M, Nakatsuka K, Morimoto I, Auron PE, Eto S. Cross-linking of intercellular adhesion molecule 1 (CD54) induces AP-1 activation and IL-1β transcription. J Immunol. 1996;157:5097–5103. [PubMed] [Google Scholar]
- Lawson C, Ainsworth M, Yacoub M, Rose M. Ligation of ICAM-1 on endothelial cells leads to expression of VCAM-1 via a nuclear factor-κB-independent mechanism. J Immunol. 1999;162:2990–2996. [PubMed] [Google Scholar]
- Husby G, Williams RC., Jr Synovial localization of tumor necrosis factor in patients with rheumatoid arthritis. J Autoimmun. 1988;1:363–371. doi: 10.1016/0896-8411(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Saxne T, Palladino MA, Jr, Heinegard D, Talal N, Wollheim FA. Detection of tumor necrosis factor α but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 1988;31:1041–1045. doi: 10.1002/art.1780310816. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Bondeson J, Brennan FM, Foxwell BM, Maini RN. The rationale for the current boom in anti-TNFα treatment. Is there an effective means to define therapeutic targets for drugs that provide all the benefits of anti-TNFα and minimise hazards? Ann Rheum Dis. 1999;58(Suppl 1):127–131. doi: 10.1136/ard.58.2008.i27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SS, Jung LK, Walters JA, Chen W, Wang CY, Fu SM. Production of tumor necrosis factor/cachectin by human T cell lines and peripheral blood T lymphocytes stimulated by phorbol myristate acetate and anti-CD3 antibody. J Exp Med. 1988;167:937–953. doi: 10.1084/jem.167.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech JT, Andreakos E, Ciesielski CJ, Green P, Foxwell BMJ, Brennan FM. T cell contact-dependent regulation of CC and CXC chemokine production in monocytes through differential involvement of NFκB: implications for rheumatoid arthritis. Arthritis Res Ther. 2006;8:168–177. doi: 10.1186/ar2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Grover BJ, Whichard LP, Hale LP, Nunley JA, McCollum DE, Singer KH. Synovial microenvironment-T cell interactions. Human T cells bind to fibroblast-like synovial cells in vitro. Arthritis Rheum. 1988;31:947–955. doi: 10.1002/art.1780310802. [DOI] [PubMed] [Google Scholar]
- Bombara MP, Webb DL, Conrad P, Marlor CW, Sarr T, Ranges GE, Aune TM, Greve JM, Blue ML. Cell contact between T cells and synovial fibroblasts causes induction of adhesion molecules and cytokines. J Leukoc Biol. 1993;54:399–406. doi: 10.1002/jlb.54.5.399. [DOI] [PubMed] [Google Scholar]
- Shier P, Ngo K, Fung-Leung WP. Defective CD8+ T cell activation and cytolytic function in the absence of LFA-1 cannot be restored by increased TCR signaling. J Immunol. 1999;163:4826–4832. [PubMed] [Google Scholar]
- Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990;144:4579–4586. [PubMed] [Google Scholar]
- Morales-Ducret J, Wayner E, Elices MJ, Alvaro-Garcia JM, Zvaifler N, Firestein GS. α4/β1 integrin (VLA-4) ligands in arthritis: vascular cell adhesion molecule-1 expression in synovium and on fibroblast-like synoviocytes. J Immunol. 1992;149:1424–1431. [PubMed] [Google Scholar]
- Cho CS, Cho ML, Min SY, Kim WU, Min DJ, Lee SS, Park SH, Choe J, Kim HY. CD40 engagement on synovial fibroblast up-regulates production of vascular endothelial growth factor. J Immunol. 2000;164:5055–5061. doi: 10.4049/jimmunol.164.10.5055. [DOI] [PubMed] [Google Scholar]
- Vallejo AN, Yang H, Klimiuk PA, Weyand CM, Goronzy JJ. Synoviocyte-mediated expansion of inflammatory T cells in rheumatoid synovitis is dependent on CD47-thrombospondin 1 interaction. J Immunol. 2003;171:1732–1740. doi: 10.4049/jimmunol.171.4.1732. [DOI] [PubMed] [Google Scholar]
- Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003;48:594–601. doi: 10.1002/art.10816. [DOI] [PubMed] [Google Scholar]
- Chu CQ, Field M, Feldmann M, Maini RN. Localization of tumor necrosis factor α in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:1125–1132. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- Brockhaus M, Schoenfeld HJ, Schlaeger EJ, Hunziker W, Lesslauer W, Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci USA. 1990;87:3127–3131. doi: 10.1073/pnas.87.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann HP, Remy R, Brockhaus M, van Loon AP. Two different cell types have different major receptors for human tumor necrosis factor (TNF α). J Biol Chem. 1989;264:14927–14934. [PubMed] [Google Scholar]
- Beutler B, Cerami A. The biology of cachectin/TNF—a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Decker T, Lohmann-Matthes ML, Gifford GE. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987;138:957–962. [PubMed] [Google Scholar]
- Perez C, Albert I, DeFay K, Zachariades N, Gooding L, Kriegler M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell. 1990;63:251–258. doi: 10.1016/0092-8674(90)90158-b. [DOI] [PubMed] [Google Scholar]
- Karp SE, Hwu P, Farber A, Restifo NP, Kriegler M, Mule JJ, Rosenberg SA. In vivo activity of tumor necrosis factor (TNF) mutants. Secretory but not membrane-bound TNF mediates the regression of retrovirally transduced murine tumor. J Immunol. 1992;149:2076–2081. [PMC free article] [PubMed] [Google Scholar]
- Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Dayer JM, Beutler B, Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985;162:2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Katsikis P, Brennan FM, Walker J, Bijl H, Ghrayeb J, Woody JN. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor α. Arthritis Rheum. 1993;36:1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Leeb B, Breedveld FC, Macfarlane JD, Bijl H, Woody JN. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Katz Y, Nadiv O, Beer Y. Interleukin-17 enhances tumor necrosis factor α-induced synthesis of interleukins 1, 6, and 8 in skin and synovial fibroblasts: a possible role as a “fine-tuning cytokine” in inflammation processes. Arthritis Rheum. 2001;44:2176–2184. doi: 10.1002/1529-0131(200109)44:9<2176::aid-art371>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- van Dullemen HM, van Deventer SJ, Hommes DW, Bijl HA, Jansen J, Tytgat GN, Woody J. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- Plevy SE, Landers CJ, Prehn J, Carramanzana NM, Deem RL, Shealy D, Targan SR. A role for TNF-α and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s disease. J Immunol. 1997;159:6276–6282. [PubMed] [Google Scholar]
- Agnholt J, Dahlerup JF, Kaltoft K. The effect of etanercept and infliximab on the production of tumour necrosis factor α, interferon-γ and GM-CSF in in vivo activated intestinal T lymphocyte cultures. Cytokine. 2003;23:76–85. doi: 10.1016/s1043-4666(03)00201-1. [DOI] [PubMed] [Google Scholar]
- Agnholt J, Kaltoft K. Infliximab downregulates interferon-γ production in activated gut T-lymphocytes from patients with Crohn’s disease. Cytokine. 2001;15:212–222. doi: 10.1006/cyto.2001.0919. [DOI] [PubMed] [Google Scholar]
- Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, van Montfrans C, Hommes DW, Peppelenbosch MP, van Deventer SJ. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn’s disease. Gastroenterology. 2003;124:1774–1785. doi: 10.1016/s0016-5085(03)00382-2. [DOI] [PubMed] [Google Scholar]
- Di Sabatino A, Ciccocioppo R, Cinque B, Millimaggi D, Morera R, Ricevuti L, Cifone MG, Corazza GR. Defective mucosal T cell death is sustainably reverted by infliximab in a caspase dependent pathway in Crohn’s disease. Gut. 2004;53:70–77. doi: 10.1136/gut.53.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezzonico RD, Burger D, Dayer JM. Direct contact between T lymphocytes and human dermal fibroblasts or synoviocytes down-regulates types I and III collagen production via cell-associated cytokines. J Biol Chem. 1998;273:18720–18728. doi: 10.1074/jbc.273.30.18720. [DOI] [PubMed] [Google Scholar]
- Burger D, Rezzonico R, Li JM, Modoux C, Pierce RA, Welgus HG, Dayer JM. Imbalance between interstitial collagenase and tissue inhibitor of metalloproteinases 1 in synoviocytes and fibroblasts upon direct contact with stimulated T lymphocytes: involvement of membrane-associated cytokines. Arthritis Rheum. 1998;41:1748–1759. doi: 10.1002/1529-0131(199810)41:10<1748::AID-ART7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.