Abstract
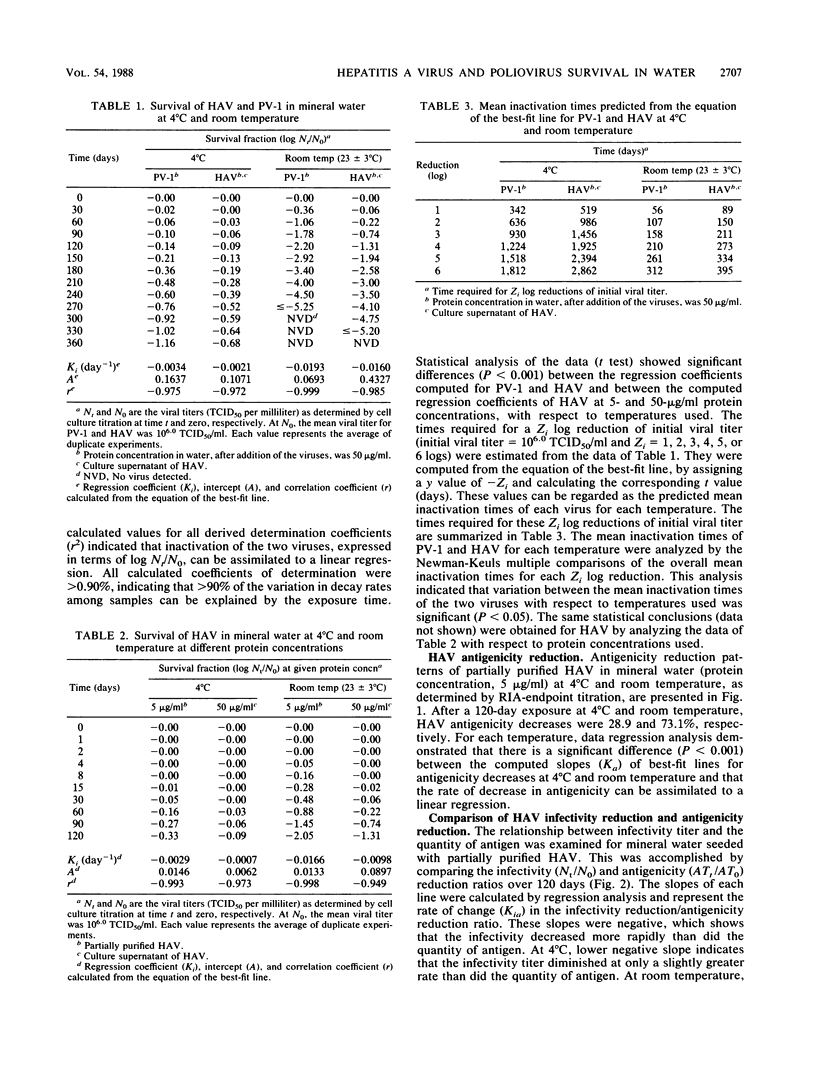
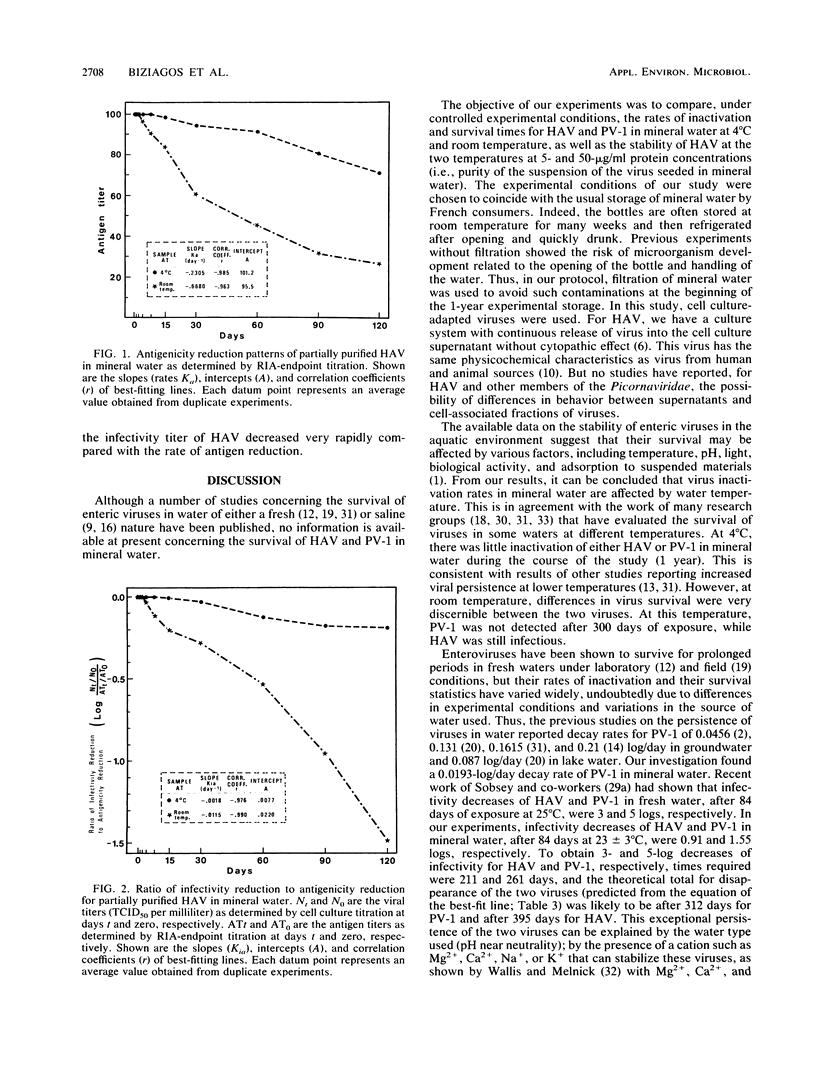
The survival in mineral water of hepatitis A virus (HAV) and poliovirus type 1 was compared, under controlled experimental conditions, at 4 degrees C and room temperature. Viral infectivity titers were determined by cell culture titration, while HAV antigenicity was monitored by radioimmunoassay-endpoint titration. Both viruses persisted longest at 4 degrees C. At this temperature, after 1 year of exposure, the inactivation of either HAV or poliovirus type 1 was not important. At room temperature, poliovirus type 1 was not detected after 300 days, whereas HAV was still infectious. For both temperatures, the computed regression coefficients of best-fit lines for inactivation rates for the two viruses were significantly different. The survival of HAV was also studied at 4 degrees C and room temperature in mineral water with 5- and 50-micrograms/ml protein concentrations (i.e., purity of the virus suspension) for 120 days. As shown by a comparison of the regression coefficients for the inactivation rates, the stability of HAV in mineral water depends on protein concentration and temperature. Radioimmunoassay-endpoint titration results showed inactivation patterns similar to those of cell culture titration, with the most significant reduction in HAV antigenicity at room temperature. At the two temperatures, the infectivity of HAV declined at a faster rate than the antigenicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biziagos E., Crance J. M., Passagot J., Deloince R. Effect of antiviral substances on hepatitis A virus replication in vitro. J Med Virol. 1987 May;22(1):57–66. doi: 10.1002/jmv.1890220108. [DOI] [PubMed] [Google Scholar]
- Crance J. M., Passagot J., Biziagos E., Deloince R. Continuous production of hepatitis A virus in PLC/PRF/5 cell cultures: use of antigen for serology. J Virol Methods. 1987 Nov;18(2-3):193–203. doi: 10.1016/0166-0934(87)90124-8. [DOI] [PubMed] [Google Scholar]
- Crance J. M., Passagot J., Verwaerde N., Biziagos E., Gandré H., Deloince R. Adaptation à la lignée cellulaire PLC/PRF/5 du virus de l'hépatite A libéré dans le surnageant de culture. C R Acad Sci III. 1985;301(7):361–363. [PubMed] [Google Scholar]
- Fujioka R. S., Loh P. C., Lau L. S. Survival of human enteroviruses in the Hawaiian ocean environment: evidence for virus-inactivating microorganisms. Appl Environ Microbiol. 1980 Jun;39(6):1105–1110. doi: 10.1128/aem.39.6.1105-1110.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust I. D., Coulepis A. G., Feinstone S. M., Locarnini S. A., Moritsugu Y., Najera R., Siegl G. Taxonomic classification of hepatitis A virus. Intervirology. 1983;20(1):1–7. doi: 10.1159/000149367. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hurst C. J., Gerba C. P., Cech I. Effects of environmental variables and soil characteristics on virus survival in soil. Appl Environ Microbiol. 1980 Dec;40(6):1067–1079. doi: 10.1128/aem.40.6.1067-1079.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst C. J., Gerba C. P. Stability of simian rotavirus in fresh and estuarine water. Appl Environ Microbiol. 1980 Jan;39(1):1–5. doi: 10.1128/aem.39.1.1-5.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lo S., Gilbert J., Hetrick F. Stability of human enteroviruses in estuarine and marine waters. Appl Environ Microbiol. 1976 Aug;32(2):245–249. doi: 10.1128/aem.32.2.245-249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeady M. L., Siak J. S., Crowell R. L. Survival of coxsackievirus B3 under diverse environmental conditions. Appl Environ Microbiol. 1979 May;37(5):972–977. doi: 10.1128/aem.37.5.972-977.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. T., Newman J. S. Inactivation of polioviruses and coxsackieviruses in surface water. Appl Environ Microbiol. 1977 Feb;33(2):334–340. doi: 10.1128/aem.33.2.334-340.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancorbo O. C., Evanshen B. G., Campbell W. F., Lambert S., Curtis S. K., Woolley T. W. Infectivity and antigenicity reduction rates of human rotavirus strain Wa in fresh waters. Appl Environ Microbiol. 1987 Aug;53(8):1803–1811. doi: 10.1128/aem.53.8.1803-1811.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry J. V., Mortimer P. P. The heat sensitivity of hepatitis A virus determined by a simple tissue culture method. J Med Virol. 1984;14(3):277–283. doi: 10.1002/jmv.1890140312. [DOI] [PubMed] [Google Scholar]
- Passagot J., Crance J. M., Biziagos E., Laveran H., Agbalika F., Deloince R. Effect of glutaraldehyde on the antigenicity and infectivity of hepatitis A virus. J Virol Methods. 1987 May;16(1-2):21–28. doi: 10.1016/0166-0934(87)90027-9. [DOI] [PubMed] [Google Scholar]
- Purcell R. H., Wong D. C., Moritsugu Y., Dienstag J. L., Routenberg J. A., Boggs J. D. A microtiter solid-phase radioimmunoassay for hepatitis A antigen and antibody. J Immunol. 1976 Feb;116(2):349–356. [PubMed] [Google Scholar]
- Siegl G., Weitz M., Kronauer G. Stability of hepatitis A virus. Intervirology. 1984;22(4):218–226. doi: 10.1159/000149554. [DOI] [PubMed] [Google Scholar]
- Simmonds R. S., Szücs G., Metcalf T. G., Melnick J. L. Persistently infected cultures as a source of hepatitis A virus. Appl Environ Microbiol. 1985 Apr;49(4):749–755. doi: 10.1128/aem.49.4.749-755.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Wallis C., Melnick J. L. Studies on the survival and fate of enteroviruses in an experimental model of a municipal solid waste landfill and leachate. Appl Microbiol. 1975 Oct;30(4):565–574. doi: 10.1128/am.30.4.565-574.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLIS C., MENICK J. L. Cationic stabilization--a new property of enteroviruses. Virology. 1962 Apr;16:504–506. doi: 10.1016/0042-6822(62)90234-9. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Ashley C. S., Moseley R. H. Heat inactivation of poliovirus in wastewater sludge. Appl Environ Microbiol. 1976 Sep;32(3):339–346. doi: 10.1128/aem.32.3.339-346.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Winston P. E. Mechanism of inactivation of enteric viruses in fresh water. Appl Environ Microbiol. 1986 Sep;52(3):450–459. doi: 10.1128/aem.52.3.450-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M. V., Gerba C. P., Kelley L. M. Virus persistence in groundwater. Appl Environ Microbiol. 1985 Apr;49(4):778–781. doi: 10.1128/aem.49.4.778-781.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


