Abstract
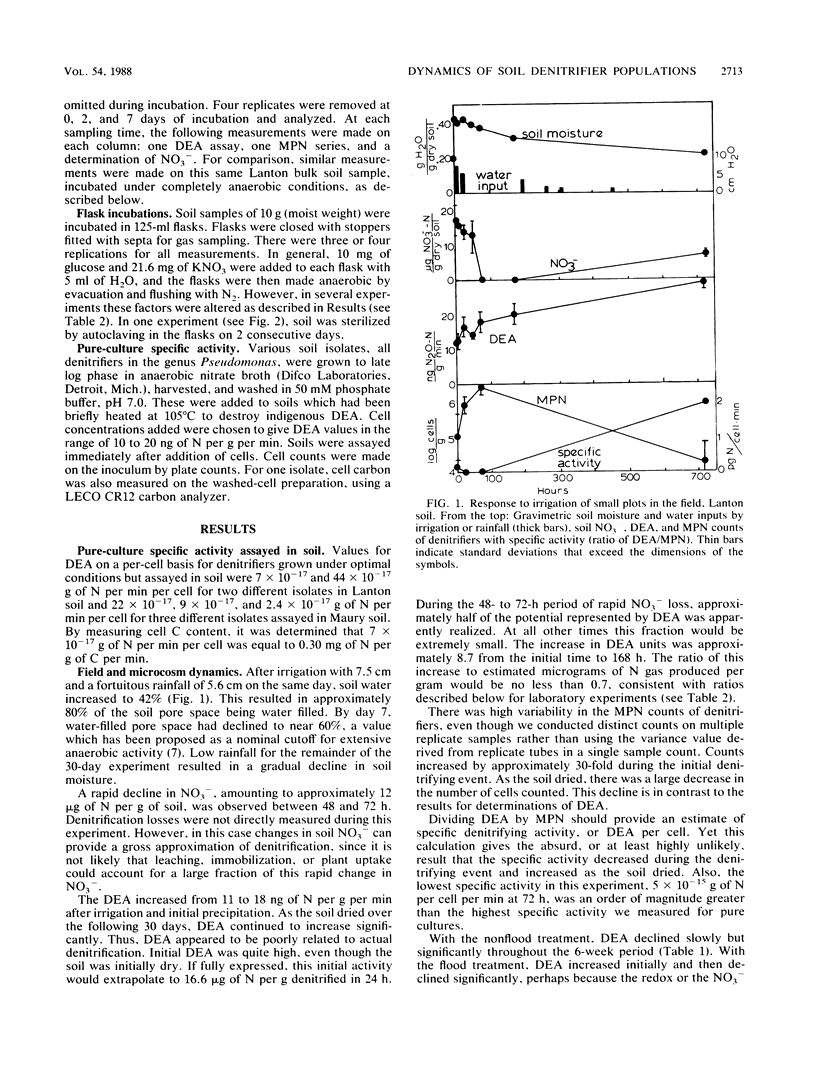
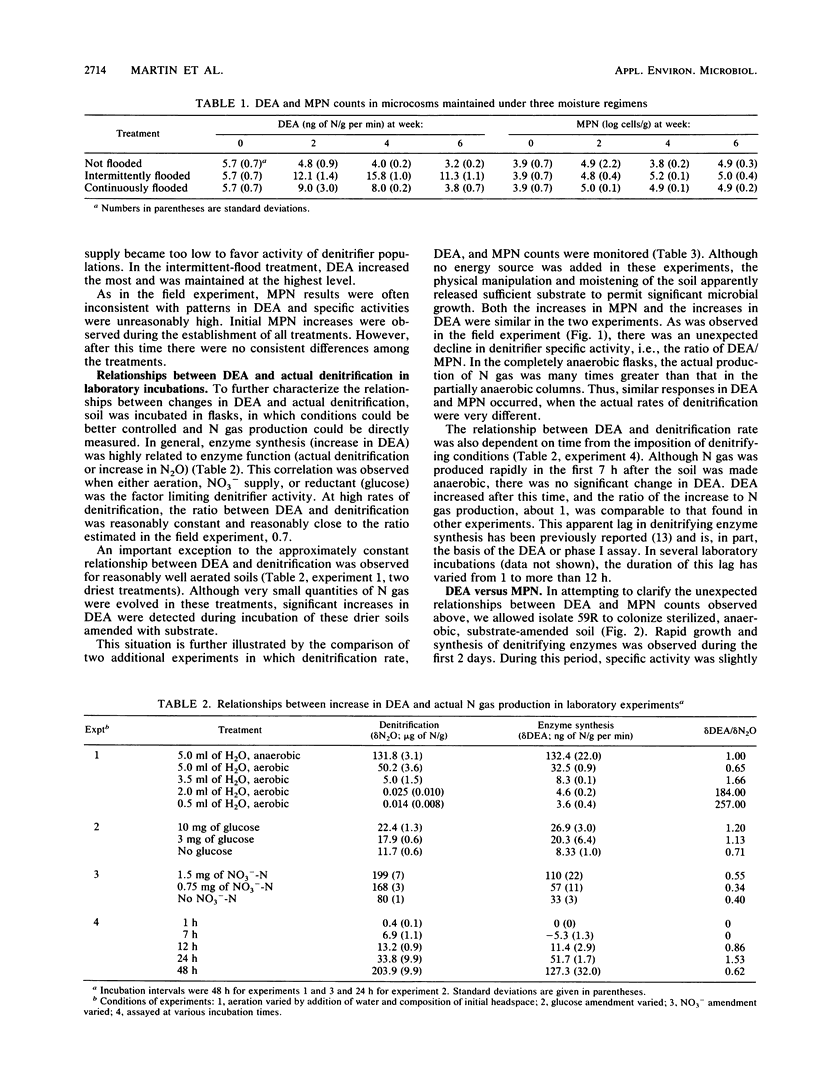
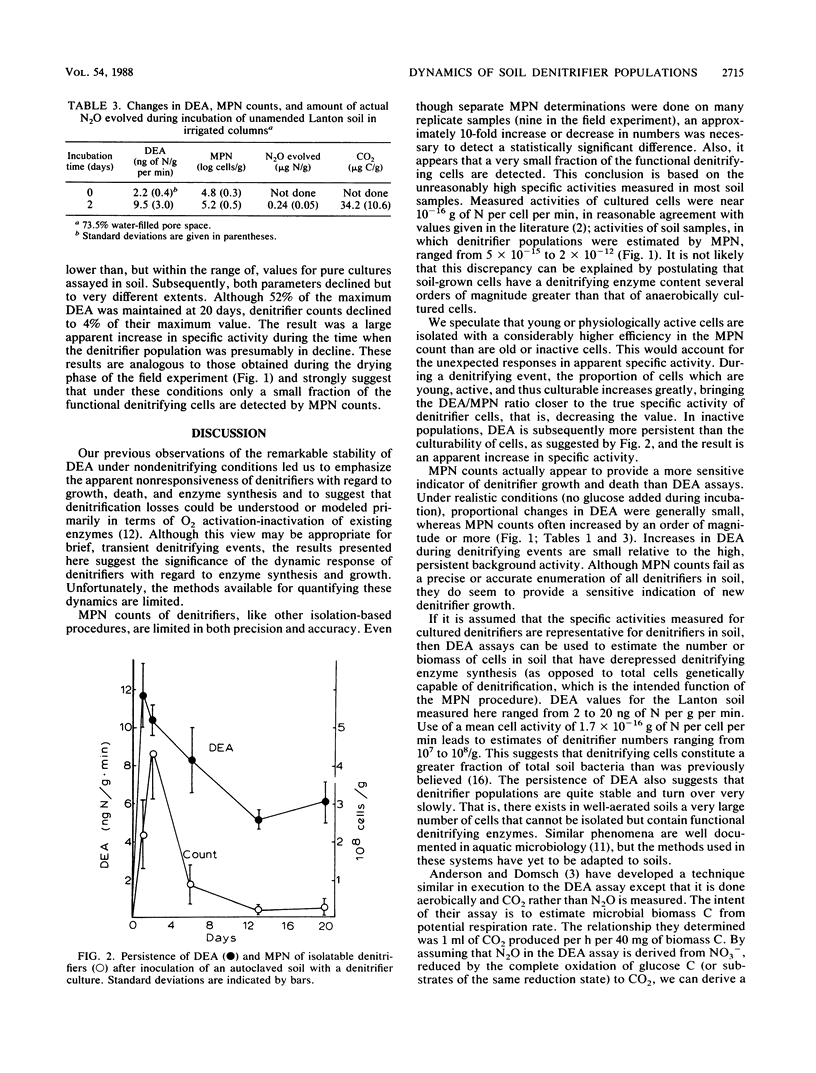
To better understand temporal variability in soil denitrification, denitrifying enzyme activity (DEA) and denitrifier populations (as determined by most-probable-number [MPN] counts) were measured in field and laboratory experiments. Measurements of DEA and MPN provided highly contradictory indications of denitrifier dynamics. In laboratory incubations, under conditions favoring active denitrification, the synthesis of new denitrifying enzymes and the actual amount of denitrification were closely related. In other experiments, however, both DEA and MPN counts were poor indicators of actual denitrification. In some cases, we found significant increases in DEA but no significant production of N gas. Except with unnaturally high substrate amendments, changes in DEA were small relative both to the persistently high DEA background and to changes in MPN. As estimated by MPN counts, denitrifier populations increased significantly during denitrification events. It was apparent that only a small fraction of the denitrifiers were included in the MPN counts, but it appeared that this isolatable fraction increased during periods of active denitrifier growth. Use of DEA as an index of biomass of cells which have synthesized denitrifying enzymes suggested that denitrifier populations were persistent, stable, and much larger than indicated by MPN procedures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Levine J. S. Relative rates of nitric oxide and nitrous oxide production by nitrifiers, denitrifiers, and nitrate respirers. Appl Environ Microbiol. 1986 May;51(5):938–945. doi: 10.1128/aem.51.5.938-945.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. S., Parsons L. L. Persistence of denitrifying enzyme activity in dried soils. Appl Environ Microbiol. 1985 Feb;49(2):316–320. doi: 10.1128/aem.49.2.316-320.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedje J. M., Sexstone A. J., Myrold D. D., Robinson J. A. Denitrification: ecological niches, competition and survival. Antonie Van Leeuwenhoek. 1982;48(6):569–583. doi: 10.1007/BF00399542. [DOI] [PubMed] [Google Scholar]


