Abstract
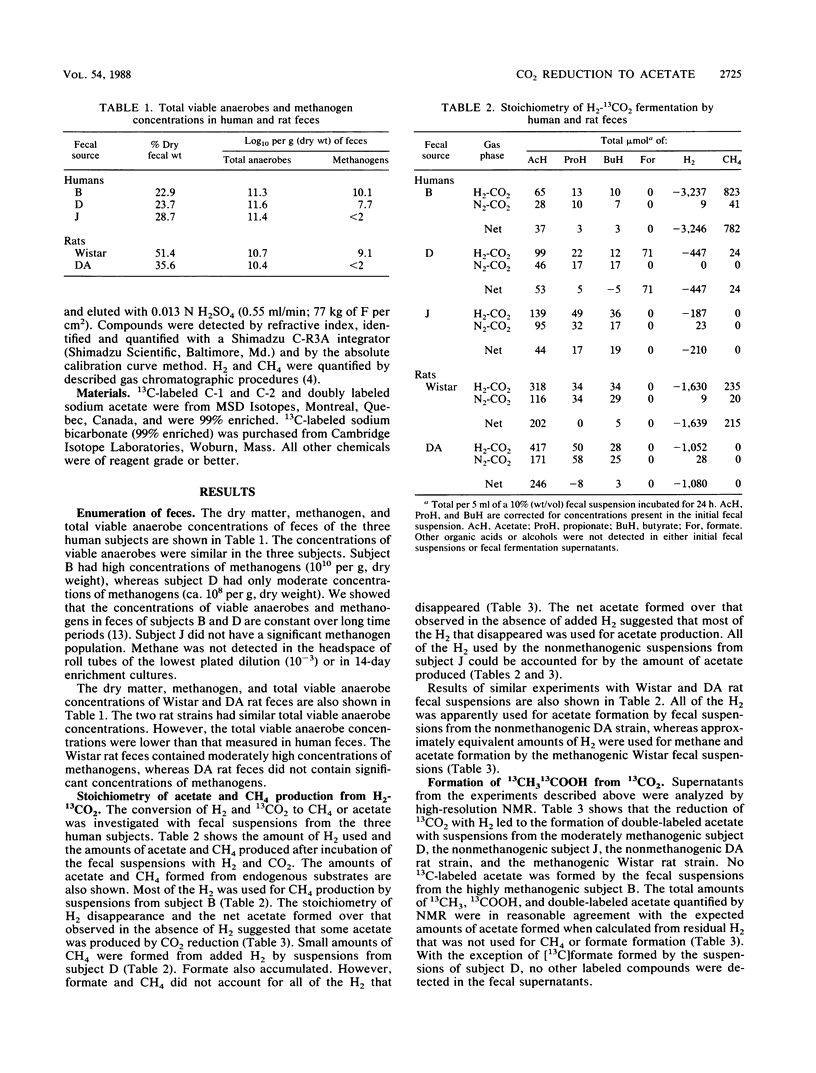
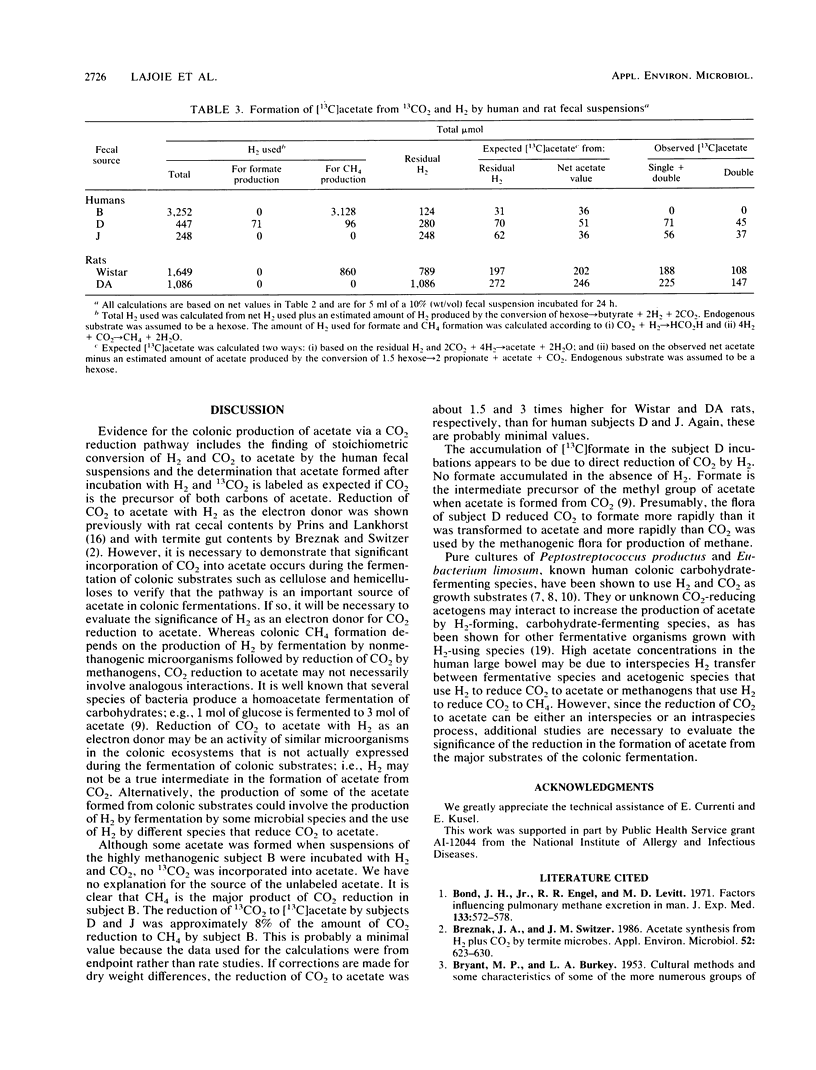
Fecal suspensions from humans were incubated with 13CO2 and H2. The suspensions were from subjects who harbored 10(8) and 10(10) methanogens per g (dry weight) of feces, respectively, and from a subject who did not harbor methanogens. Quantitative nuclear magnetic resonance spectroscopy showed that acetate labeled in both the methyl and carboxyl groups was formed by suspensions from the subject without methanogens and the subject with the lower concentrations of methanogens. The amounts of labeled acetate formed were in agreement with the amounts expected based on measurements of H2 utilization. No labeled acetate was formed by suspensions from the subject with the higher concentrations of methanogens, and essentially all of the H2 used was accounted for by CH4 production. Suspensions from the subject with lower concentrations of methanogens produced both methane and acetate from H2 and CO2. The results indicate that reduction of CO2 to acetate may be a major pathway for microbial production of acetate in the human colon except when very high concentrations of methanogens (ca. 10(10) per g [dry weight] of feces) are present. Double-labeled acetate was also formed from H2 and 13CO2 by fecal suspensions from nonmethanogenic and moderately methanogenic rats.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond J. H., Jr, Engel R. R., Levitt M. D. Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. J Exp Med. 1971 Mar 1;133(3):572–588. doi: 10.1084/jem.133.3.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznak J. A., Switzer J. M. Acetate Synthesis from H(2) plus CO(2) by Termite Gut Microbes. Appl Environ Microbiol. 1986 Oct;52(4):623–630. doi: 10.1128/aem.52.4.623-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Wolin M. J. Influence of CH4 production by Methanobacterium ruminantium on the fermentation of glucose and lactate by Selenomonas ruminantium. Appl Environ Microbiol. 1977 Dec;34(6):756–759. doi: 10.1128/aem.34.6.756-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich G. G., Goerlitz D. F., Bourell J. H., Eisen G. V., Godsy E. M. Liquid chromatographic procedure for fermentation product analysis in the identification of anaerobic bacteria. Appl Environ Microbiol. 1981 Nov;42(5):878–885. doi: 10.1128/aem.42.5.878-885.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Davis C. L., Bryant M. P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol. 1981 Jul;42(1):12–19. doi: 10.1128/aem.42.1.12-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Lorowitz W. H., Bryant M. P. Peptostreptococcus productus strain that grows rapidly with CO as the energy source. Appl Environ Microbiol. 1984 May;47(5):961–964. doi: 10.1128/aem.47.5.961-964.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Enumeration of Methanobrevibacter smithii in human feces. Arch Microbiol. 1982 Feb;131(1):14–18. doi: 10.1007/BF00451492. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Methanosphaera stadtmaniae gen. nov., sp. nov.: a species that forms methane by reducing methanol with hydrogen. Arch Microbiol. 1985 Mar;141(2):116–122. doi: 10.1007/BF00423270. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Stability of Methanobrevibacter smithii populations in the microbial flora excreted from the human large bowel. Appl Environ Microbiol. 1983 Jan;45(1):317–318. doi: 10.1128/aem.45.1.317-318.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver G. A., Krause J. A., Miller T. L., Wolin M. J. Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut. 1986 Jun;27(6):698–704. doi: 10.1136/gut.27.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw A., Sleath K. Myth of the marsupial mother: home care of very low birth weight babies in Bogota, Colombia. Lancet. 1985 May 25;1(8439):1206–1208. doi: 10.1016/s0140-6736(85)92877-6. [DOI] [PubMed] [Google Scholar]


