Abstract
1 The stimulatory effects of neurotensin (NT) and several NT fragments were evaluated in two pharmacological preparations: rat stomach strips and isolated spontaneously beating atria of guinea-pigs.
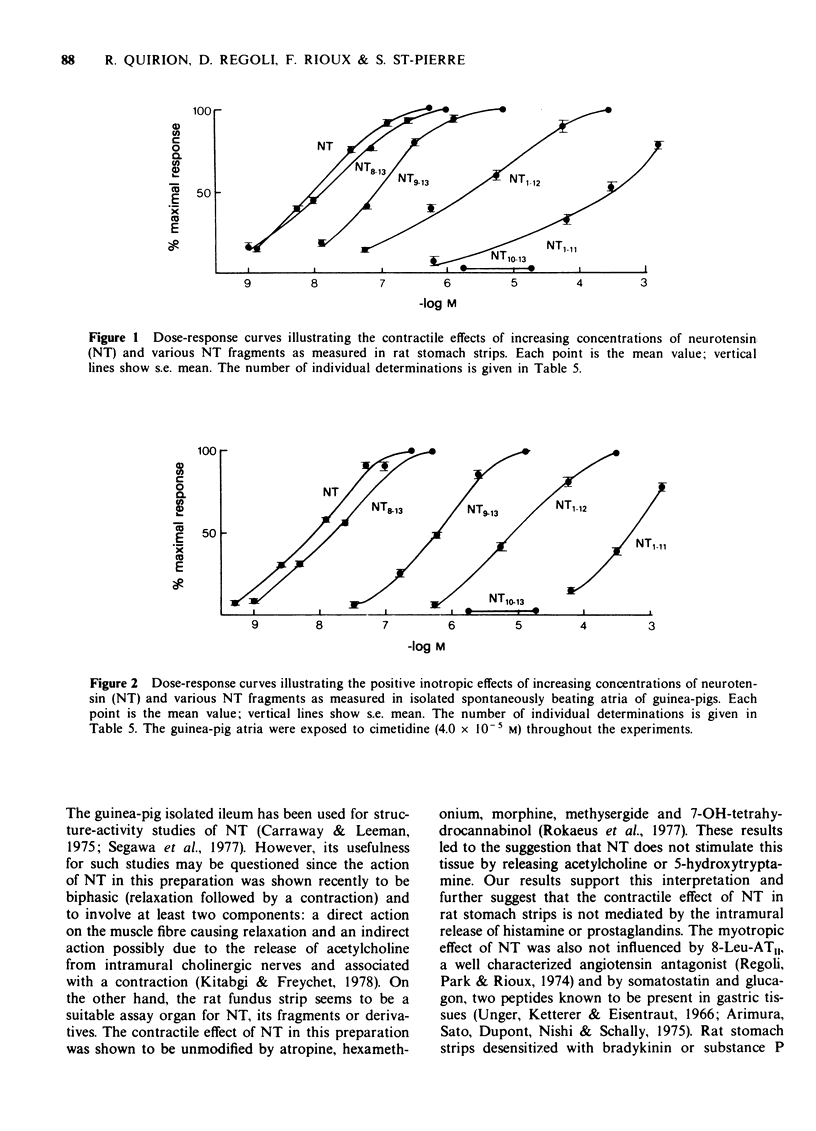
2 In rat stomach strips, NT elicited a dose-dependent contractile effect in concentrations varying between 1.3 × 10-9 and 5.4 × 10-7 M.
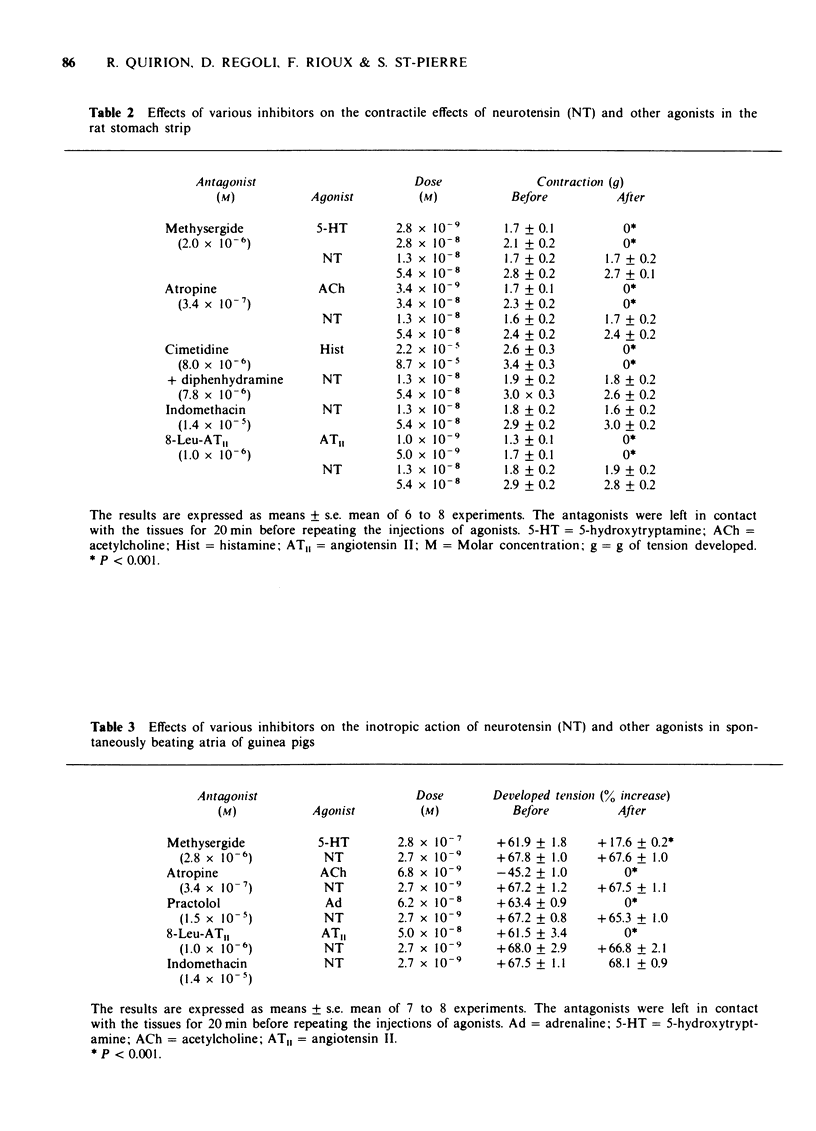
3 The contractile effect of NT (1.3 and 5.4 × 10-8 M) in this tissue was not modified by atropine (3.4 × 10-7 M), methysergide (2.0 × 10-6 M), a mixture of cimetidine (8.0 × 10-6 M) and diphenhydramine (7.8 × 10-6 M), indomethacin (1.4 × 10-5 M), 8-Leu-angiotensin II (1.0 × 10-6 M), glucagon (2.0 × 10-6 M) or somatostatin (3.0 × 10-7 M).
4 Rat stomach strips desensitized by bradykinin (6.1 × 10-6 M) or substance P (7.4 × 10-6 M) maintained their sensitivities to NT (1.3 and 5.4 × 10-8 M).
5 In guinea-pig atria, NT produced a dose-dependent positive inotropic action in concentrations varying between 5.4 × 10-10 and 2.7 × 10-7 M.
6 The inotropic effect of NT (2.7 × 10-9 M) was not influenced by methysergide (2.8 × 10-6 M), atropine (3.4 × 10-7 M), practolol (1.5 × 10-5 M), 8-Leu-angiotensin II (1.0 × 10-6 M), or indomethacin (1.4 × 10-5 M), but it was reduced by 37% by cimetidine (4.0 × 10-5 and 2.0 × 10-4 M). A combination of cimetidine (4.0 × 10-5 M) and diphenhydramine (3.9 × 10-6 M) did not produce a greater inhibition of NT than cimetidine alone.
7 Atria desensitized by bradykinin (6.1 × 10-6 M) or glucagon (2.0 × 10-6 M) maintained their sensitivities to NT (2.7 × 10-9 M). Substance P was inactive both as an agonist or antagonist of NT.
8 These results suggest the existence of specific NT receptors in rat stomach strips and guinea-pig atria.
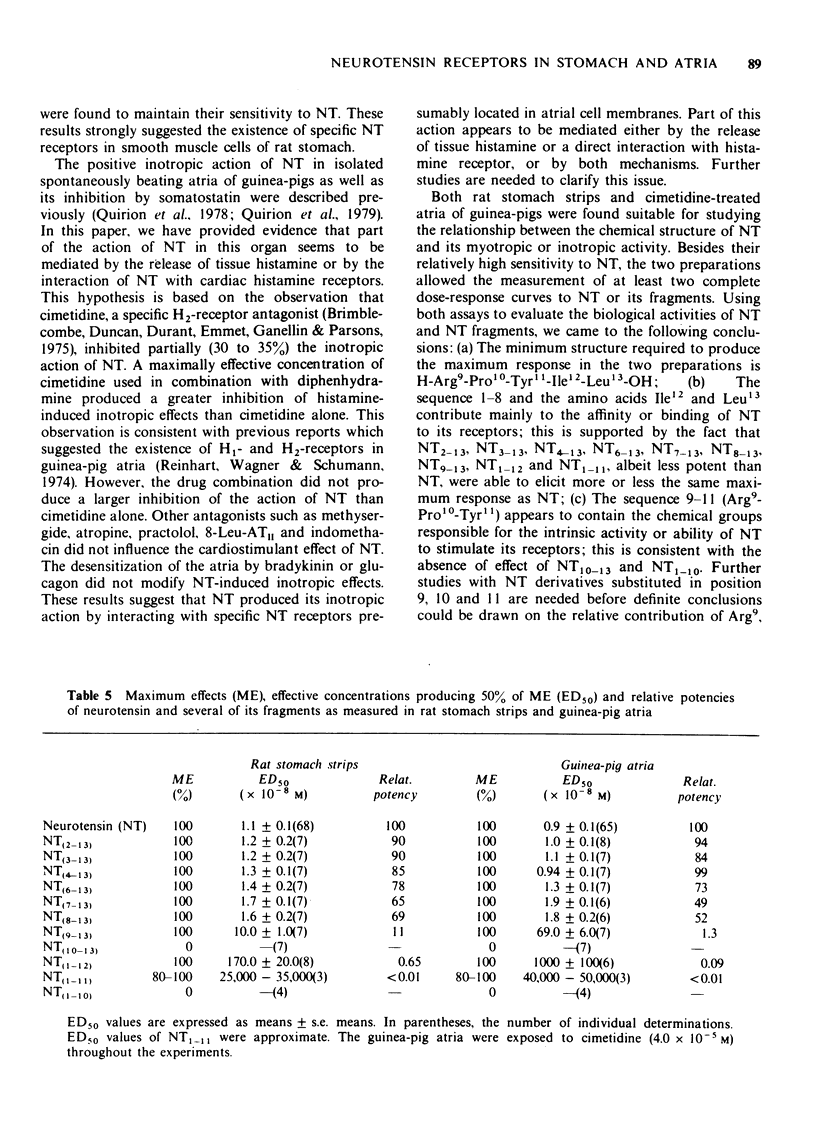
9 The data derived from our structure-activity study suggest that the minimum structure required for the full stimulation of NT receptors in these two preparations is H-Arg9-Pro10-Tyr11-Ile12-Leu13-OH. The sequence PyroGlu1-Leu2-Tyr3-Glu4-Asn5-Lys6-Pro7-Arg8- and the amino acids Ile12 and Leu13 appear to contribute mainly to the affinity or binding of NT to its receptor. The chemical groups responsible for the full activation (intrinsic activity) of NT receptors seem to be located in the sequence -Arg9-Pro10-Tyr11.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimura A., Sato H., Dupont A., Nishi N., Schally A. V. Somatostatin: abundance of immunoreactive hormone in rat stomach and pancreas. Science. 1975 Sep 19;189(4207):1007–1009. doi: 10.1126/science.56779. [DOI] [PubMed] [Google Scholar]
- Besterman H. S., Bloom S. R., Sarson D. L., Blackburn A. M., Johnston D. I., Patel H. R., Stewart J. S., Modigliani R., Guerin S., Mallinson C. N. Gut-hormone profile in coeliac disease. Lancet. 1978 Apr 15;1(8068):785–788. doi: 10.1016/s0140-6736(78)92994-x. [DOI] [PubMed] [Google Scholar]
- Bissette G., Manberg P., Nemeroff C. B., Prange A. J., Jr Neurotensin, a biologically active peptide. Life Sci. 1978 Nov 27;23(22):2173–2182. doi: 10.1016/0024-3205(78)90201-1. [DOI] [PubMed] [Google Scholar]
- Carraway R., Leeman S. E. Characterization of radioimmunoassayable neurotensin in the rat. Its differential distribution in the central nervous system, small intestine, and stomach. J Biol Chem. 1976 Nov 25;251(22):7045–7052. [PubMed] [Google Scholar]
- Carraway R., Leeman S. E. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973 Oct 10;248(19):6854–6861. [PubMed] [Google Scholar]
- Kitabgi P., Freychet P. Effects of neurotensin on isolated intestinal smooth muscles. Eur J Pharmacol. 1978 Aug 15;50(4):349–357. doi: 10.1016/0014-2999(78)90140-1. [DOI] [PubMed] [Google Scholar]
- Kobayashi R. M., Brown M., Vale W. Regional distribution of neurotensin and somatostatin in rat brain. Brain Res. 1977 May 13;126(3):584–588. doi: 10.1016/0006-8993(77)90613-8. [DOI] [PubMed] [Google Scholar]
- Loosen P. T., Nemeroff C. B., Bissette G., Burnett G. B., Prange A. J., Jr, Lipton M. A. Neurotensin-induced hypothermia in the rat: structure-activity studies. Neuropharmacology. 1978 Feb;17(2):109–113. doi: 10.1016/0028-3908(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Mashford M. L., Nilsson G., Rökaeus A., Rosell S. The effect of food ingestion on circulating neurotensin-like immunoreactivity (NTLI) in the human. Acta Physiol Scand. 1978 Oct;104(2):244–246. doi: 10.1111/j.1748-1716.1978.tb06275.x. [DOI] [PubMed] [Google Scholar]
- Orci L., Baetens O., Rufener C., Brown M., Vale W., Guillemin R. Evidence for immunoreactive neurotensin in dog intestinal mucosa. Life Sci. 1976 Aug 15;19(4):559–561. doi: 10.1016/0024-3205(76)90236-8. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Sullivan S. N., Bloom S. R., Buchan A. M., Facer P., Brown M. R., Pearse A. G. Specific localisation of neurotensin to the N cell in human intestine by radioimmunoassay and immunocytochemistry. Nature. 1977 Nov 10;270(5633):183–184. doi: 10.1038/270183a0. [DOI] [PubMed] [Google Scholar]
- Quirion R., Regoli D., Rioux F., St-Pierre S. An analysis of the negative inotropic action of somatostatin. Br J Pharmacol. 1979 Jun;66(2):251–257. doi: 10.1111/j.1476-5381.1979.tb13673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirion R., Rioux F., Regoli D. Chronotropic and inotropic effects of neurotensin on spontaneously beating auricles. Can J Physiol Pharmacol. 1978 Aug;56(4):671–673. doi: 10.1139/y78-108. [DOI] [PubMed] [Google Scholar]
- Regoli D., Park W. K., Rioux F. Pharmacology of angiotensin. Pharmacol Rev. 1974 Jun;26(2):69–123. [PubMed] [Google Scholar]
- Reinhardt D., Wagner J., Schümann H. J. Differentiation of H1- and H2-receptors mediating positive chrono- and inotropic responses to histamine on atrial preparations of the guinea-pig. Agents Actions. 1974 Oct;4(4):217–221. doi: 10.1007/BF01965222. [DOI] [PubMed] [Google Scholar]
- Rivier J. E., Lazarus L. H., Perrin M. H., Brown M. R. Neurotensin analogues. Structure--activity relationships. J Med Chem. 1977 Nov;20(11):1409–1412. doi: 10.1021/jm00221a011. [DOI] [PubMed] [Google Scholar]
- Rökaeus A., Burcher E., Chang D., Folkers K., Rosell S. Actions of neurotensin and (Gln4)-neurotensin on isolated tissues. Acta Pharmacol Toxicol (Copenh) 1977 Aug;41(2):141–147. doi: 10.1111/j.1600-0773.1977.tb02134.x. [DOI] [PubMed] [Google Scholar]
- Segawa T., Hosokawa M., Kitagawa K., Yajima H. Contractile activity of synthetic neurotensin and related polypeptides on guinea-pig ileum. J Pharm Pharmacol. 1977 Jan;29(1):57–58. doi: 10.1111/j.2042-7158.1977.tb11243.x. [DOI] [PubMed] [Google Scholar]
- Uhl G. R., Kuhar M. J., Snyder S. H. Neurotensin: immunohistochemical localization in rat central nervous system. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4059–4063. doi: 10.1073/pnas.74.9.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Eisentraut A. M. Distribution of immunoassayable glucagon in gastrointestinal tissues. Metabolism. 1966 Oct;15(10):865–867. doi: 10.1016/0026-0495(66)90156-9. [DOI] [PubMed] [Google Scholar]
- VANE J. R. A sensitive method for the assay of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1957 Sep;12(3):344–349. doi: 10.1111/j.1476-5381.1957.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]


