Abstract
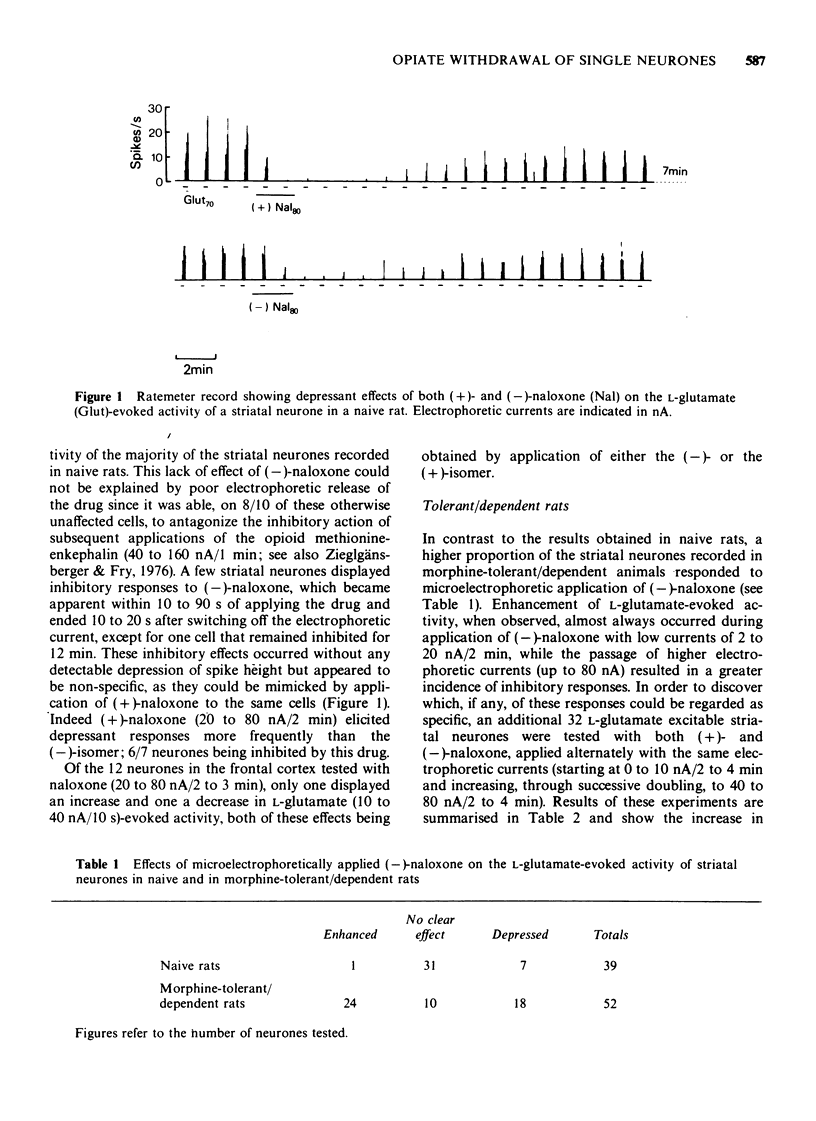
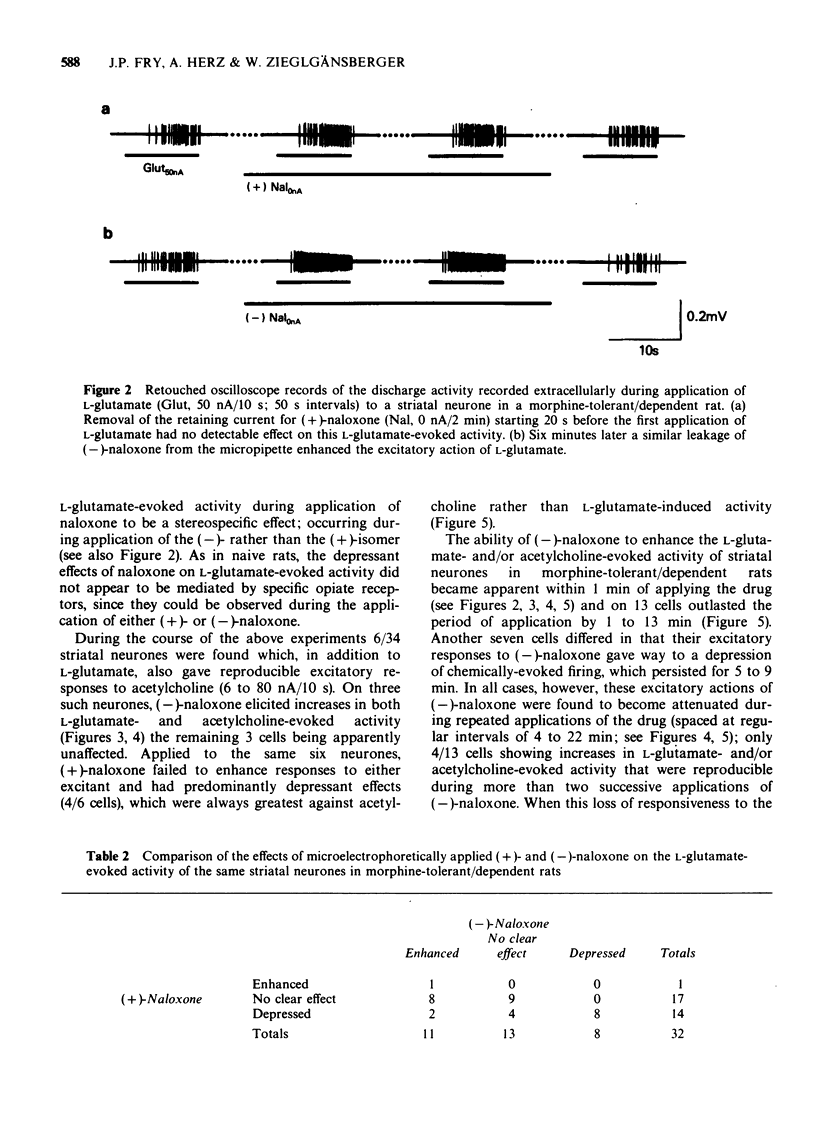
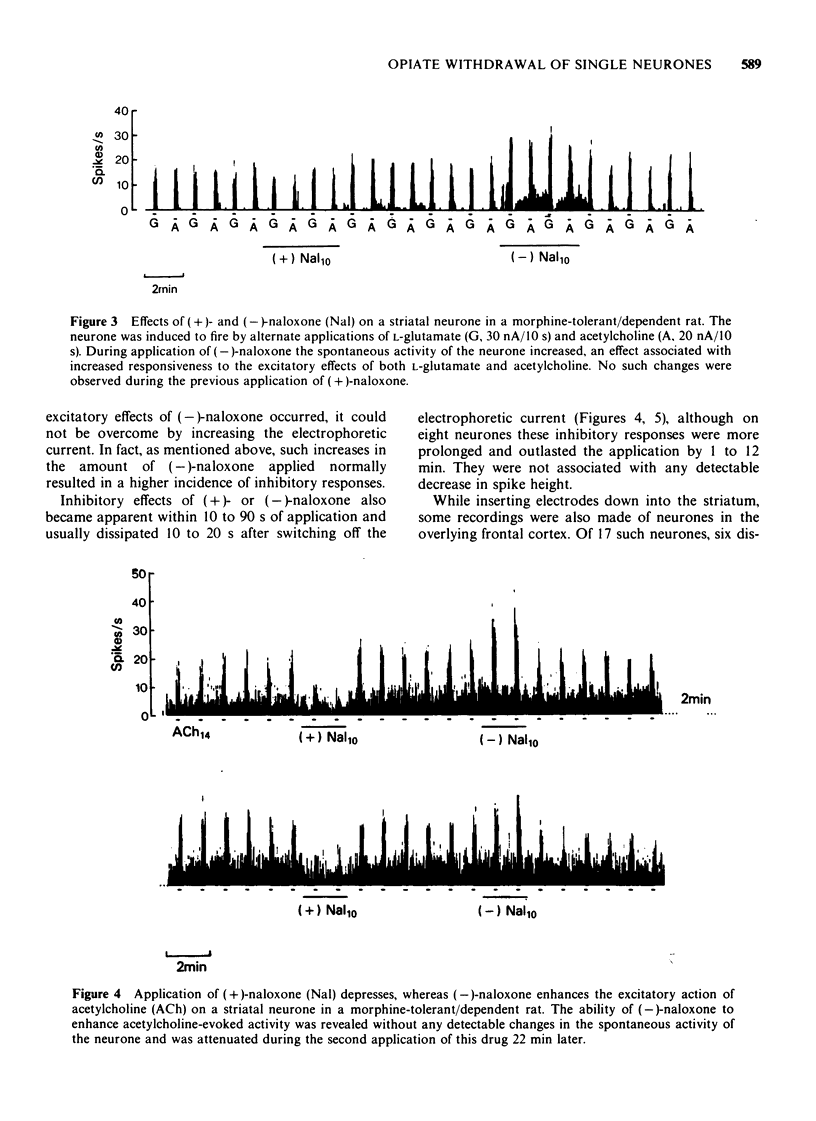
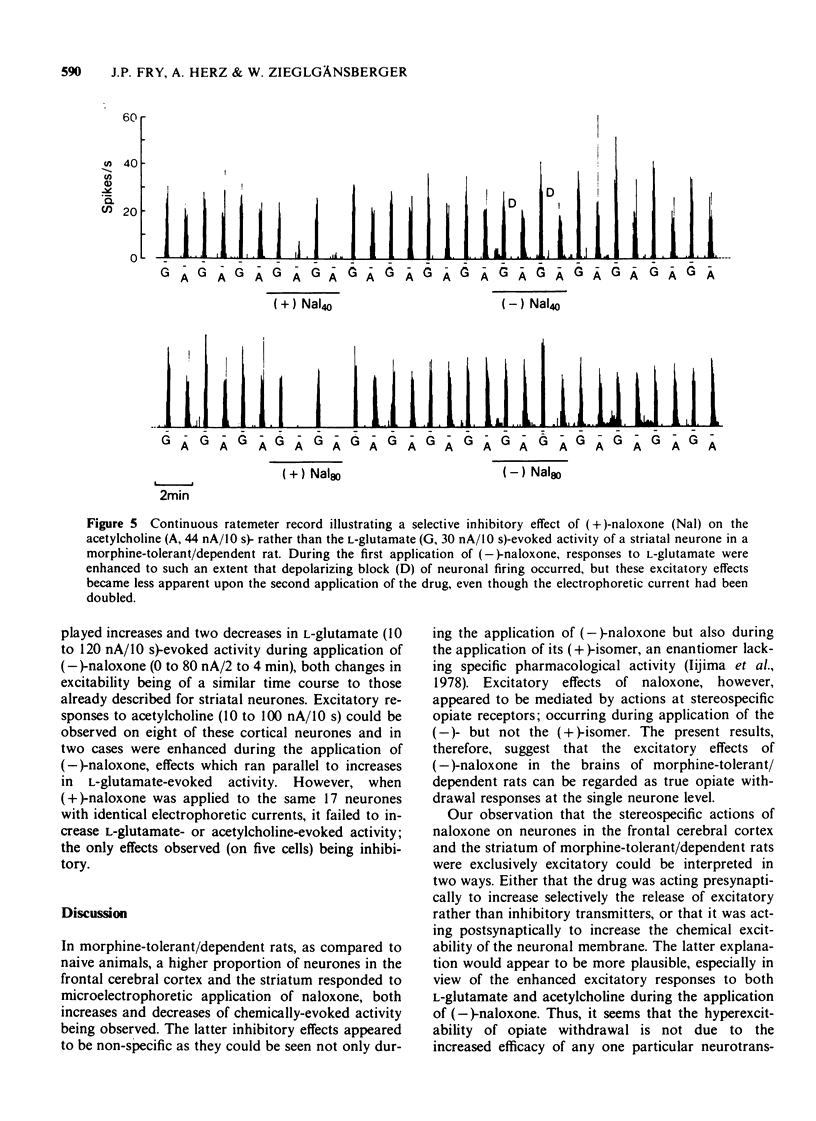
1 A comparison has been made between the effects of microelectrophoretically applied naloxone on single neurones in the frontal cerebral cortex and the striatum of naive and of morphine-tolerant/dependent rats, anaesthetized with a mixture of alpha-chloralose and urethane. 2 Specificity of the results obtained was evaluated by contrasting the effects of alternate applications of the (+)- and (-)-isomers of naloxone to the same neurones. 3 In naive rats naloxone had predominantly no effect, only a few cells revealing non-specific depressant responses to the drug. 4 In morphine-tolerant/dependent rats a higher proportion of neurones responded to naloxone; either with stereospecific excitatory responses, in which the activity evoked by L-glutamate or acetylcholine was increased, or with a non-specific inhibition, similar to that observed in naive animals. 5 It is suggested that these excitatory responses to microelectrophoretically applied (-)-naloxone represent opiate withdrawal responses at the single neurone level and that they reflect a latent hyperexcitability of the postsynaptic membrane in the morphine-tolerant/dependent state.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978 Nov 9;276(5684):186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- Bläsig J., Herz A., Reinhold K., Zieglgänsberger S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia. 1973 Oct 23;33(1):19–38. doi: 10.1007/BF00428791. [DOI] [PubMed] [Google Scholar]
- COLLIER H. O. A GENERAL THEORY OF THE GENESIS OF DRUG DEPENDENCE BY INDUCTION OF RECEPTORS. Nature. 1965 Jan 9;205:181–182. doi: 10.1038/205181a0. [DOI] [PubMed] [Google Scholar]
- Davies J. Effects of morphine and naloxone on Renshaw cells and spinal interneurones in morphine dependent and non-dependent rats. Brain Res. 1976 Aug 27;113(2):311–326. doi: 10.1016/0006-8993(76)90943-4. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Davies J., Hall J. G. Effects of opiate agonists and antagonists on central neurons of the cat. J Pharmacol Exp Ther. 1976 Jan;196(1):107–120. [PubMed] [Google Scholar]
- Frederickson R. C., Norris F. H. Enkephalin-induced depression of single neurons in brain areas with opiate receptors--antagonism by naloxone. Science. 1976 Oct 22;194(4263):440–442. doi: 10.1126/science.10625. [DOI] [PubMed] [Google Scholar]
- Frederickson R. C., Norris F. H., Hewes C. R. Effects of naloxone and acetylcholine on medial thalamic and cortical units in naive and morphine dependent rats. Life Sci. 1975 Jul 1;17(1):81–82. doi: 10.1016/0024-3205(75)90240-4. [DOI] [PubMed] [Google Scholar]
- Fry J. P., Zieglgänsberger W., Herz A. Specific versus non-specific actions of opioids on hippocampal neurones in the rat brain. Brain Res. 1979 Mar 16;163(2):295–305. doi: 10.1016/0006-8993(79)90357-3. [DOI] [PubMed] [Google Scholar]
- Gayton R. J., Lambert L. A., Bradley P. B. Failure of (+)-naloxone to antagonise responses to opioid peptides. Neuropharmacology. 1978 Jul;17(7):549–551. doi: 10.1016/0028-3908(78)90063-1. [DOI] [PubMed] [Google Scholar]
- Iijima I., Minamikawa J., Jacobson A. E., Brossi A., Rice K. C. Studies in the (+)-morphinan series. 5. Synthesis and biological properties of (+)-naloxone. J Med Chem. 1978 Apr;21(4):398–400. doi: 10.1021/jm00202a018. [DOI] [PubMed] [Google Scholar]
- Jaffe J. H., Sharpless S. K. XVII. Pharmacological denervation supersensitivity in the central nervous system: a theory of physical dependence. Res Publ Assoc Res Nerv Ment Dis. 1968;46:226–246. [PubMed] [Google Scholar]
- Johnson S. M., Westfall D. P., Howard S. A., Fleming W. W. Sensitivities of the isolated ileal longitudinal smooth muscle-myenteric plexus and hypogastric nerve-vas deferens of the guinea pig after chronic morphine pellet implantation. J Pharmacol Exp Ther. 1978 Jan;204(1):54–66. [PubMed] [Google Scholar]
- Kosterlitz H. W., Watt A. J. Kinetic parameters of narcotic agonists and antagonists, with particular reference to N-allylnoroxymorphone (naloxone). Br J Pharmacol Chemother. 1968 Jun;33(2):266–276. doi: 10.1111/j.1476-5381.1968.tb00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Martin W. R. XVI. A homeostatic and redundancy theory of tolerance to and dependence on narcotic analgesics. Res Publ Assoc Res Nerv Ment Dis. 1968;46:206–225. [PubMed] [Google Scholar]
- Nicoll R. A., Siggins G. R., Ling N., Bloom F. E., Guillemin R. Neuronal actions of endorphins and enkephalins among brain regions: a comparative microiontophoretic study. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2584–2588. doi: 10.1073/pnas.74.6.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Karras P. J. Opiate tolerance and dependence induced in vitro in single myenteric neurones. Nature. 1978 Mar 2;272(5648):73–75. doi: 10.1038/272073a0. [DOI] [PubMed] [Google Scholar]
- North R. A., Zieglgänsberger W. Opiate withdrawal signs in single myenteric neurones. Brain Res. 1978 Apr 7;144(1):208–211. doi: 10.1016/0006-8993(78)90453-5. [DOI] [PubMed] [Google Scholar]
- Satoh M., Zieglgänsberger W., Herz A. Actions of opiates upon single unit activity in the cortex of naive and tolerant rats. Brain Res. 1976 Oct 8;115(1):99–110. doi: 10.1016/0006-8993(76)90825-8. [DOI] [PubMed] [Google Scholar]
- Schulz R., Herz A. Aspects of opiate dependence in the myenteric plexus of the guinea-pig. Life Sci. 1976 Oct 15;19(8):1117–1127. doi: 10.1016/0024-3205(76)90246-0. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Klee W. A., Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3092–3096. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber J., Gullis R., Hamprecht B. Influence of opiates on the levels of adenosine 3':5'-cyclic monophosphate in neuroblastoma X glioma hybrid cells. Life Sci. 1975 Jun 15;16(12):1863–1868. doi: 10.1016/0024-3205(75)90292-1. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Bayerl H. The mechanism of inhibition of neuronal activity by opiates in the spinal cord of cat. Brain Res. 1976 Oct 8;115(1):111–128. doi: 10.1016/0006-8993(76)90826-x. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Puil E. A. Actions of glutamic acid on spinal neurones. Exp Brain Res. 1973 Mar 29;17(1):35–49. doi: 10.1007/BF00234562. [DOI] [PubMed] [Google Scholar]


