Abstract
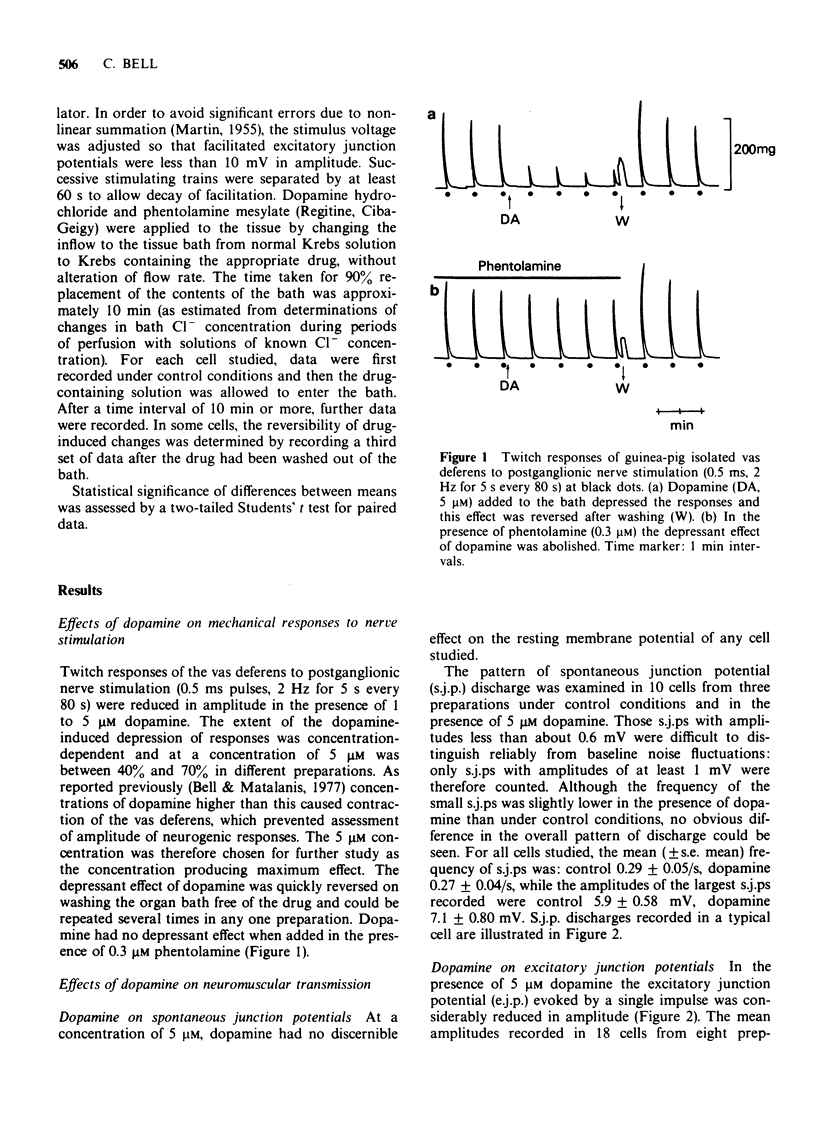
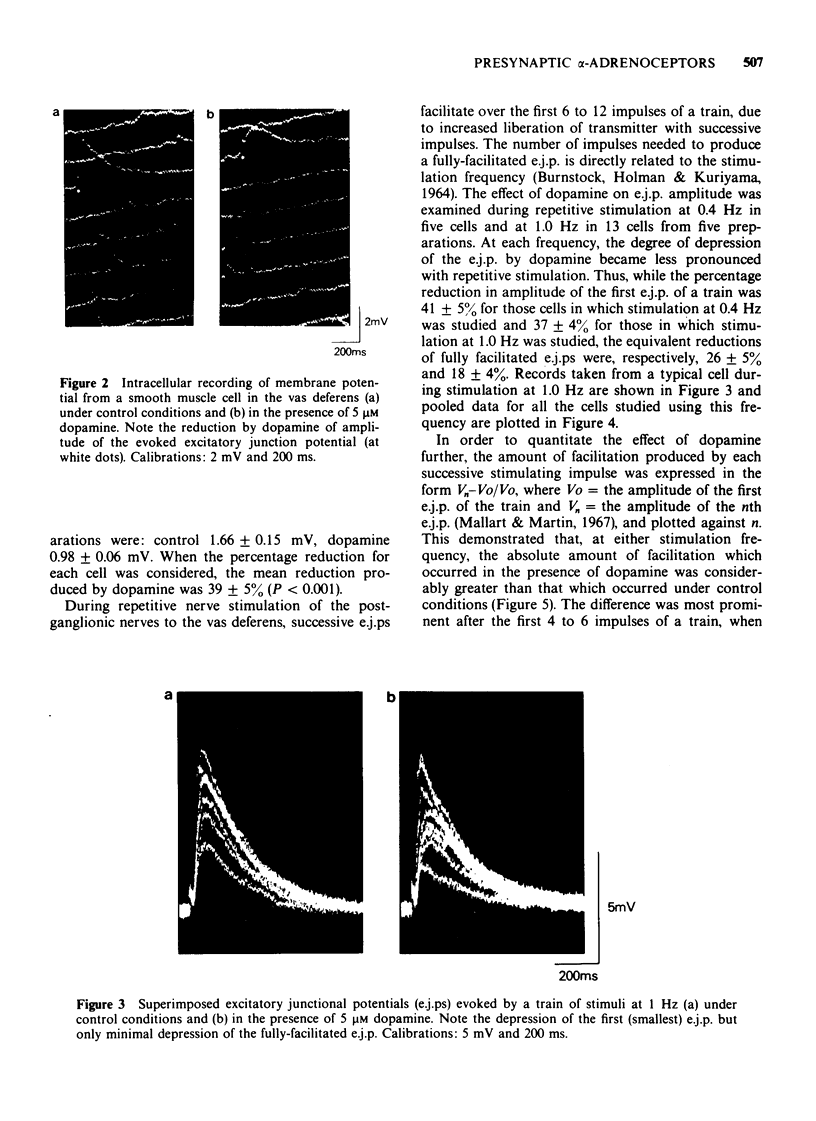
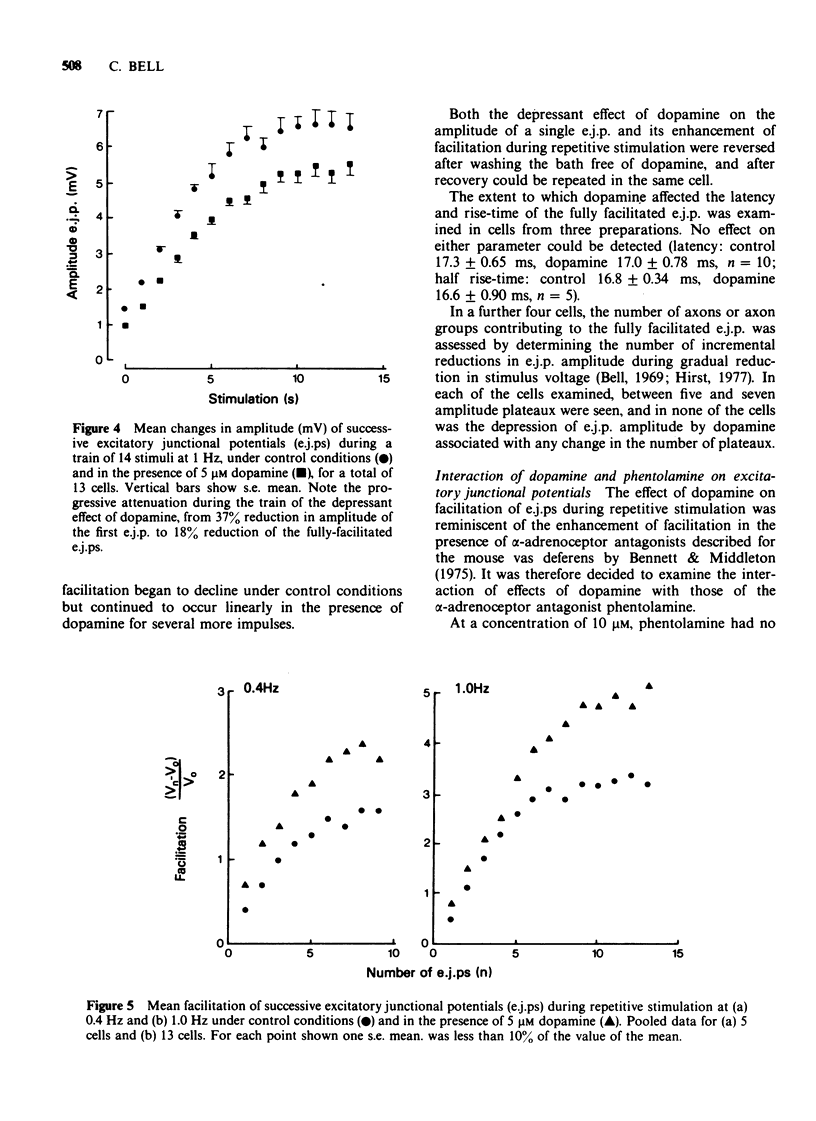
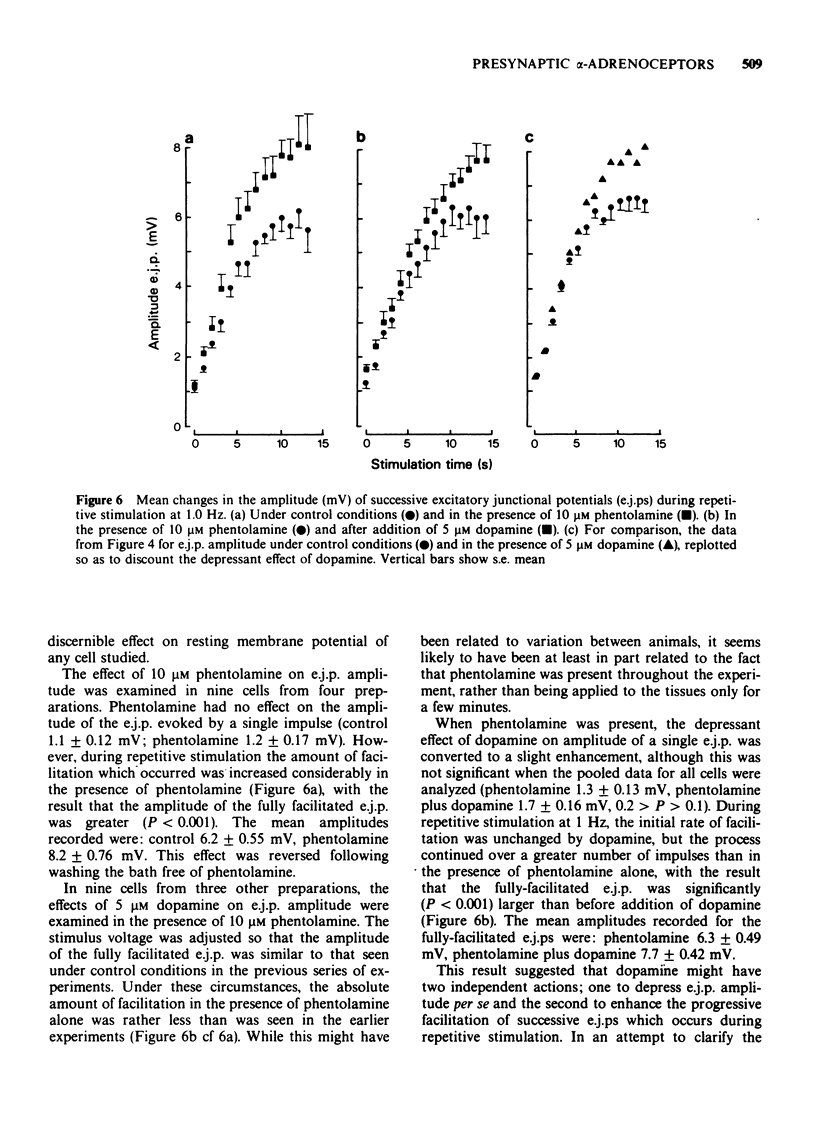
1 In the isolated vas deferens of the guinea-pig, contractile responses to adrenergic nerve stimulation at 2 Hz were depressed by exogenous dopamine (5 microM) and this effect was abolished in the presence of phentolamine (0.3 microM), suggesting that it was due to an agonist action of dopamine on alpha-adrenotors. 2 The depression by dopamine (5 microM) of contractile responses to nerve stimulation was correlated with reduction in amplitude of single excitatory junction potentials (e.j.ps) evoked by nerve stimulation, but not with depression of spontaneous junction potentials. 3 By contrast, during repetitive nerve stimulation at 1 Hz the depressant effect of dopamine on e.j.p. amplitude became less pronounced, due to the amount of facilitation being greater than that occurring under control conditions in the same cell. 4 The alpha-adrenoceptor antagonist, phentolamine (10 microM), also increased the amount of facilitation during repetitive nerve stimulation. 5 In the presence of phentolamine (10 microM), the depressant effect of dopamine (5 microM) on single e.j.ps was abolished but its enhancing effect on facilitation was not reduced. 6 It is suggested that the enhancement of facilitation during repetitive stimulation by both dopamine and phentolamine is independent of their actions on presynaptic alpha-adrenoceptors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Zar M. A. Evidence against adrenergic motor transmission in the guinea-pig vas deferens. J Physiol. 1971 Jul;216(2):359–389. doi: 10.1113/jphysiol.1971.sp009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. M., Boura A. L. Effects of clonidine and guanethidine on peripheral sympathetic nerve function in the pithed rat. Br J Pharmacol. 1973 Apr;47(4):850–852. doi: 10.1111/j.1476-5381.1973.tb08214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN G. L., GILLESPIE J. S. The output of sympathetic transmitter from the spleen of the cat. J Physiol. 1957 Aug 29;138(1):81–102. doi: 10.1113/jphysiol.1957.sp005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. Effect of denervation and of reserpine treatment on transmission at sympathetic nerve endings. J Physiol. 1962 Mar;160:461–469. doi: 10.1113/jphysiol.1962.sp006859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E., KURIYAMA H. FACILITATION OF TRANSMISSION FROM AUTONOMIC NERVE TO SMOOTH MUSCLE OF GUINEA-PIG VAS DEFERENS. J Physiol. 1964 Jul;172:31–49. doi: 10.1113/jphysiol.1964.sp007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. Spontaneous potential at sympathetic nerve endings in smooth muscle. J Physiol. 1962 Mar;160:446–460. doi: 10.1113/jphysiol.1962.sp006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. Depression of adrenergic neuromuscular transmission by presynaptic alpha-adrenoceptor activation [proceedings]. J Physiol. 1978 Nov;284:134P–134P. [PubMed] [Google Scholar]
- Bell C., Matalanis G. Dopamine-induced depression of adrenergic nerve-mediated contraction of smooth muscle. Br J Pharmacol. 1977 Oct;61(2):291–295. doi: 10.1111/j.1476-5381.1977.tb08418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. Transmission from vasoconstrictor and vasodilator nerves to single smooth muscle cells of the guinea-pig uterine artery. J Physiol. 1969 Dec;205(3):695–708. doi: 10.1113/jphysiol.1969.sp008991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. An electrophysiological analysis of the storage and release of noradrenaline at sympathetic nerve terminals. J Physiol. 1973 Mar;229(2):515–531. doi: 10.1113/jphysiol.1973.sp010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Middleton J. An electrophysiological analysis of the effects of amine-uptake blockers and alpha-adrenoceptor blockers on adrenergic neuromuscular transmission. Br J Pharmacol. 1975 Sep;55(1):87–95. doi: 10.1111/j.1476-5381.1975.tb07615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N., Nishi S. Effects of dopamine on the superior cervical ganglion of the rabbit. J Physiol. 1974 May;239(1):155–164. doi: 10.1113/jphysiol.1974.sp010560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enero M. A., Langer S. Z., Rothlin R. P., Stefano F. J. Role of the -adrenoceptor in regulating noradrenaline overflow by nerve stimulation. Br J Pharmacol. 1972 Apr;44(4):672–688. doi: 10.1111/j.1476-5381.1972.tb07306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnebo L. O., Hamberger B. Drug-induced changes in the release of ( 3 H)-noradrenaline from field stimulated rat iris. Br J Pharmacol. 1971 Sep;43(1):97–106. doi: 10.1111/j.1476-5381.1971.tb07160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., WILLIS W. D. Hyperpolarization of mammalian motor nerve terminals. J Physiol. 1962 Aug;163:115–137. doi: 10.1113/jphysiol.1962.sp006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUKOVIC S. Responses of the isolated sympathetic nerveductus deferens preparation of the guinea-pig. Br J Pharmacol Chemother. 1961 Apr;16:188–194. doi: 10.1111/j.1476-5381.1961.tb00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977 Dec;273(1):263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Willis W. D. The effects of depolarization of motor nerve terminals upon the release of transmitter by nerve impulses. J Physiol. 1968 Feb;194(2):381–405. doi: 10.1113/jphysiol.1968.sp008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. Evaluation of mechanisms controlling the release and inactivation of the adrenergic transmitter in the rabbit portal vein and vas deferens. Br J Pharmacol. 1972 Mar;44(3):472–491. doi: 10.1111/j.1476-5381.1972.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst M. J., Marshall I., Nasmyth P. A. Dopamine inhibition of the twitch response of the mouse isolated vas deferens [proceedings]. Br J Pharmacol. 1979 May;66(1):131P–131P. [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Some effects produced by adrenaline upon neuromuscular propagation in rats. J Physiol. 1958 Apr 30;141(2):291–304. doi: 10.1113/jphysiol.1958.sp005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Puig M. Effect of flow-stop on noradrenaline release from normal spleens and spleens treated with cocaine, phentolamine or phenoxybenzamine. Br J Pharmacol. 1971 Oct;43(2):359–369. [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of catecholamine release. Biochem Pharmacol. 1974 Jul 1;23(13):1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- Langer S. Z. The metabolism of (3H)noradrenaline released by electrical stimulation from the isolated nictitating membrane of the cat and from the vas deferens of the rat. J Physiol. 1970 Jul;208(3):515–546. doi: 10.1113/jphysiol.1970.sp009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K. Alpha sympathomimetic inhibition of adrenergic and cholinergic transmission in the rabbit heart. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(1):18–45. doi: 10.1007/BF00501004. [DOI] [PubMed] [Google Scholar]
- Starke K., Endo T., Taube H. D. Relative pre- and postsynaptic potencies of alpha-adrenoceptor agonists in the rabbit pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol. 1975;291(1):55–78. doi: 10.1007/BF00510821. [DOI] [PubMed] [Google Scholar]
- Starke K. Influence of -receptor stimulants on noradrenaline release. Naturwissenschaften. 1971 Aug;58(8):420–420. doi: 10.1007/BF00591535. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Vizi E. S., Somogyi G. T., Hadházy P., Knoll J. Effect of duration and frequency of stimulation on the presynaptic inhibition by alpha-adrenoceptor stimulation of the adrenergic transmission. Naunyn Schmiedebergs Arch Pharmacol. 1973;280(1):79–91. doi: 10.1007/BF00505357. [DOI] [PubMed] [Google Scholar]
- Westfall T. C. Local regulation of adrenergic neurotransmission. Physiol Rev. 1977 Oct;57(4):659–728. doi: 10.1152/physrev.1977.57.4.659. [DOI] [PubMed] [Google Scholar]


