Abstract
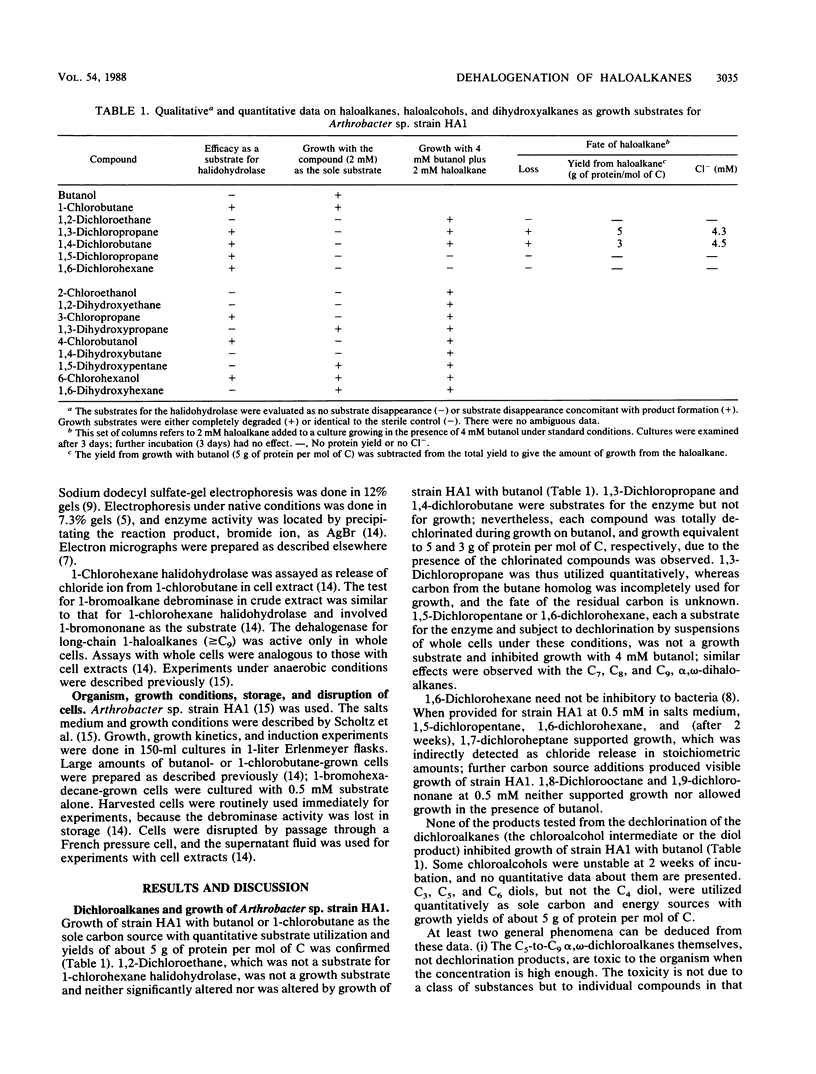
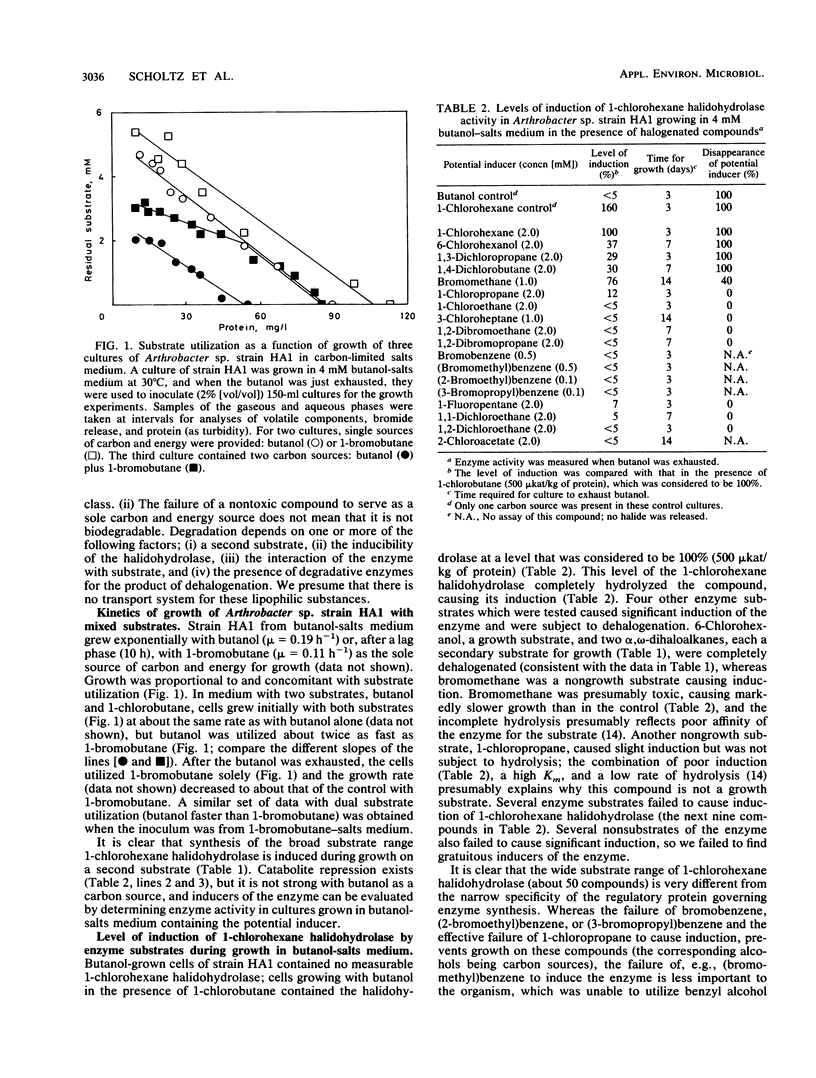
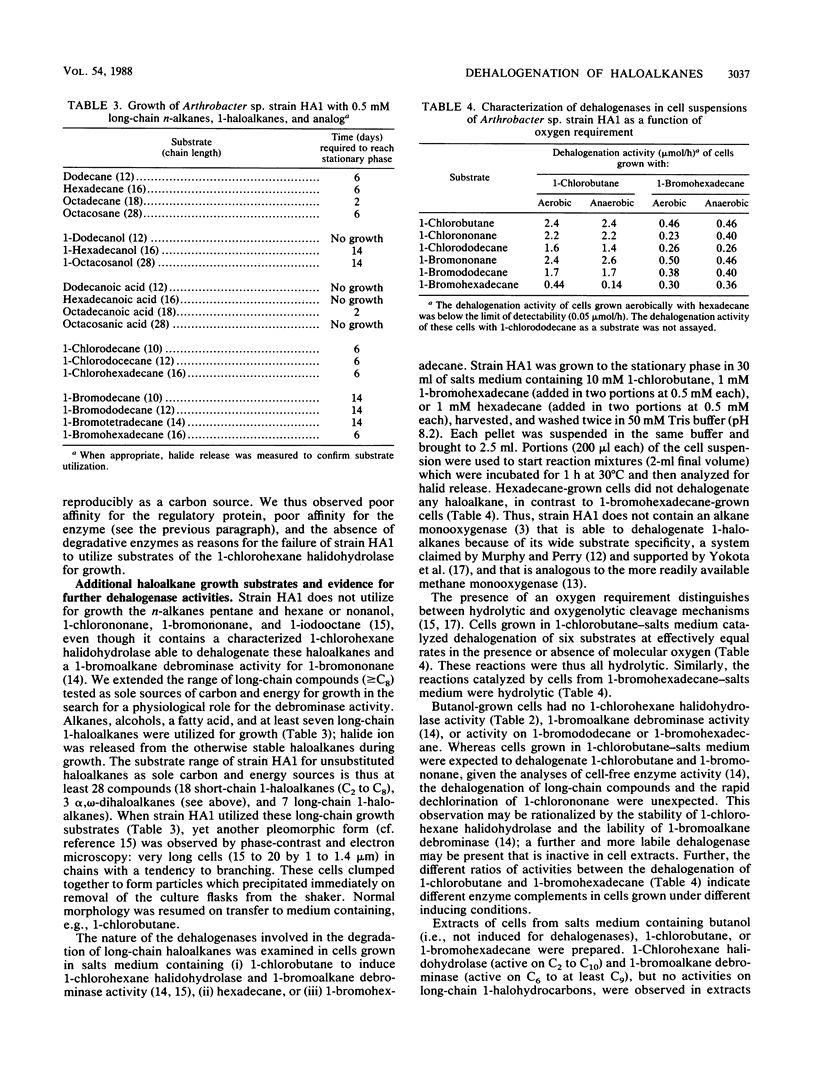
Arthrobacter sp. strain HA1 utilizes 18 C2-to-C8 1-haloalkanes for growth and synthesizes an inducible 1-bromoalkane debrominase of unknown physiological function (R. Scholtz, T. Leisinger, F. Suter, and A.M. Cook, J. Bacteriol. 169:5016-5021, 1987) in addition to an inducible 1-chlorohexane halidohydrolase which dehalogenates some 50 substrates, including alpha, omega-dihaloalkanes. alpha, omega-Dihaloalkanes were utilized by cultures of strain HA1 under certain conditions only. C9 and C8 homologs prevented growth. At suitable concentrations, C7-to-C5 homologs could serve as sole sources of carbon and energy for growth. C4 and C3 homologs could be utilized only in the presence of a second substrate (e.g., butanol), and the C2 homolog was not degraded. Kinetics of growth and substrate utilization indicated that cells of strain HA1 growing in butanol-salts medium could be used to test whether compounds induced the 1-chlorohexane halidohydrolase. No gratuitous induction of synthesis of the enzyme was observed. Many enzyme substrates (e.g., bromobenzene) did not induce synthesis of the enzyme, though the enzyme sequence to degrade the product (phenol) was present. Some inducers (e.g., bromomethane) were enzyme substrates but not growth substrates. In an attempt to find a physiological role for the 1-bromoalkane debrominase, we observed that several long-chain haloaliphatic compounds (greater than C9; e.g., 1-bromohexadecane and 1-chlorohexadecane) were utilized for growth and that induced cells could dehalogenate several 1-haloalkanes (at least C4 to C16). The dehalogenation of the long-chain compounds could not be assayed in the cell extract, so we presume that a third haloalkane dehalogenase was present.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Janssen D. B., Jager D., Witholt B. Degradation of n-haloalkanes and alpha, omega-dihaloalkanes by wild-type and mutants of Acinetobacter sp. strain GJ70. Appl Environ Microbiol. 1987 Mar;53(3):561–566. doi: 10.1128/aem.53.3.561-566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Motosugi K., Soda K. Microbial degradation of synthetic organochlorine compounds. Experientia. 1983 Nov 15;39(11):1214–1220. doi: 10.1007/BF01990358. [DOI] [PubMed] [Google Scholar]
- Murphy G. L., Perry J. J. Chlorinated fatty acid distribution in Mycobacterium convolutum phospholipids after growth on 1-chlorohexadecane. Appl Environ Microbiol. 1987 Jan;53(1):10–13. doi: 10.1128/aem.53.1.10-13.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Felix A. Microbial Oxidation of Hydrocarbons: Properties of a Soluble Methane Monooxygenase from a Facultative Methane-Utilizing Organism, Methylobacterium sp. Strain CRL-26. Appl Environ Microbiol. 1982 Nov;44(5):1130–1137. doi: 10.1128/aem.44.5.1130-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtz R., Leisinger T., Suter F., Cook A. M. Characterization of 1-chlorohexane halidohydrolase, a dehalogenase of wide substrate range from an Arthrobacter sp. J Bacteriol. 1987 Nov;169(11):5016–5021. doi: 10.1128/jb.169.11.5016-5021.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


