Abstract
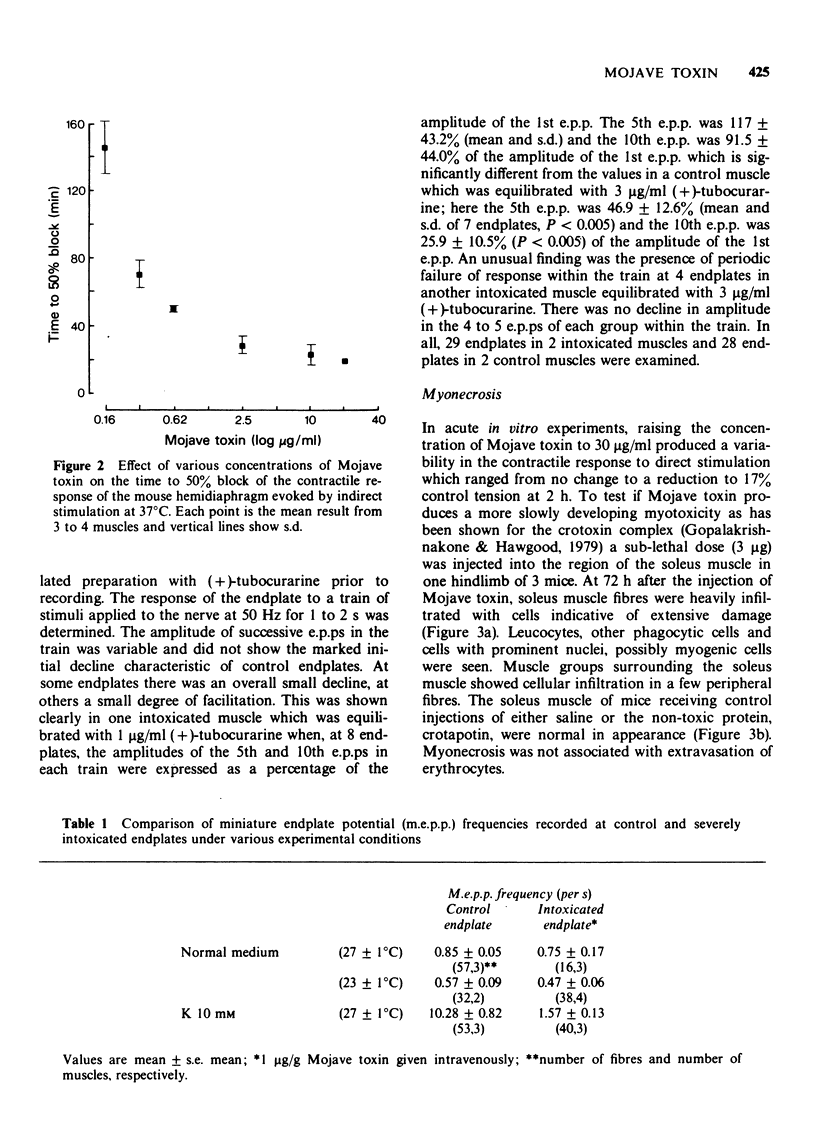
1 Mojave toxin isolated from the venom of the Mojave rattlesnake (Crotalus scutulatus scutulatus) produced an irreversible blockade of the contractile response of the mouse hemidiaphragm to stimulation of the phrenic nerve in vitro, at concentrations of 0.16 to 20 μg/ml; the response to direct stimulation was not affected over a testing period of several hours.
2 Mojave toxin (1 to 4 μg/g) was injected into the tail vein of mice and the intoxicated hemidiaphragm preparation was removed either for testing the contractile response or for intracellular recording.
3 In fully intoxicated hemidiaphragms the contractile response to indirect stimulation was either small and transient or absent, whilst the response to direct stimulation was well maintained.
4 Intracellular recording showed that resting membrane potentials of the muscle fibres were within the normal range. Endplates were difficult to locate but miniature endplate potentials (m.e.p.ps) were recorded at sites at which neurally evoked responses either could not be detected or did not exceed 2 mV which corresponds to transmitter release of a few quanta only.
5 The mean frequency of m.e.p.ps at fully intoxicated endplates was not significantly different from controls but potassium depolarization produced only a small increase in m.e.p.p. frequency relative to the control response. A 50 Hz tetanus had no effect on m.e.p.p. frequency.
6 When a sub-lethal dose (3 μg) of Mojave toxin was injected into one hindlimb of mice and the tissues examined at 72 h, there was histological evidence of myonecrosis.
7 The isolated perfused heart of the rat was exposed to recycled Mojave toxin (50 and 100 μg/ml) but showed no change in rate or force of ventricular contraction.
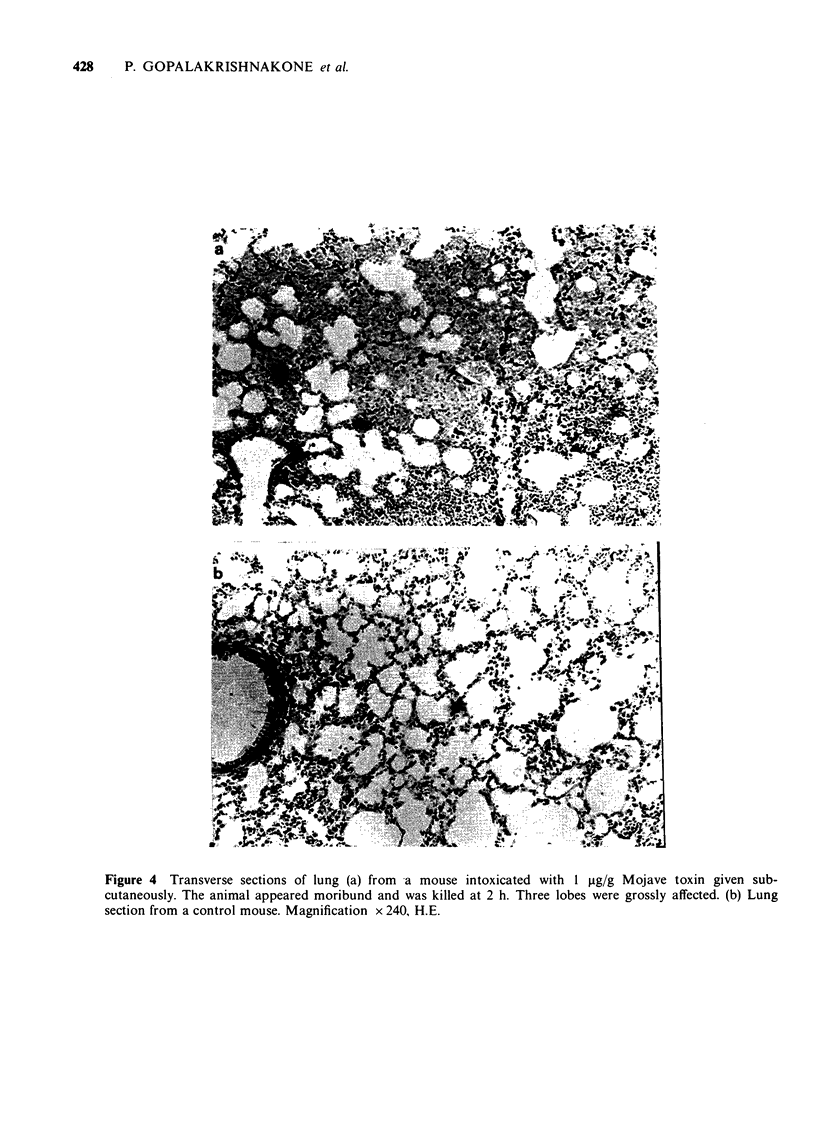
8 Post-mortem examination of intoxicated mice showed a frequent incidence of localized areas of interstitial and intra-alveolar haemorrhage in the lungs. Other organs including skin and muscle were not affected.
9 Mojave toxin showed antigenic similarities to crotoxin, the lethal neurotoxin in the venom of the South American rattlesnake, as determined by the ability of antiserum raised against crotoxin to neutralize Mojave toxin.
10 With systemic Mojave intoxication of rapid onset, the cause of death was respiratory paralysis. However, the toxin acts at multiple sites at differing rates of action. With a slower rate of intoxication, impaired respiration may act synergistically with cardiovascular changes to produce circulatory failure. The desirability of using an antivenin with a high titre against Mojave toxin is indicated.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieber A. L., Tu T., Tu A. T. Studies of an acidic cardiotoxin isolated from the venom of Mojave rattlesnake (Crotalus scutulatus). Biochim Biophys Acta. 1975 Jul 21;400(1):178–188. doi: 10.1016/0005-2795(75)90139-7. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Flower R. J., Nijkamp F. P., Vane J. R. Phospholipase A2 activity of guinea-pig perfused lungs: stimulation and inhibition by anti-inflammatory steroids [proceedings]. Br J Pharmacol. 1977 Mar;59(3):441P–441P. [PMC free article] [PubMed] [Google Scholar]
- Brazil O. V., Fariña R., Yoshida L., De Oliveira V. A. Pharmacology of crystalline crotoxin. 3. Cardiovascular and respiratory effects of crotoxin and Crotalus durissus terrificus venom. Mem Inst Butantan. 1966;33(3):993–1000. [PubMed] [Google Scholar]
- Brazil O. V. Neurotoxins from the South American rattle snake venom. Taiwan Yi Xue Hui Za Zhi. 1972 Jul 28;71(6):394–400. [PubMed] [Google Scholar]
- Breithaupt H. Neurotoxic and myotoxic effects of crotalus phospholipase A and its complex with crotapotin. Naunyn Schmiedebergs Arch Pharmacol. 1976;292(3):271–278. doi: 10.1007/BF00517389. [DOI] [PubMed] [Google Scholar]
- Cate R. L., Bieber A. L. Purification and characterization of Mojave (Crotalus scutulatus scutulatus) toxin and its subunits. Arch Biochem Biophys. 1978 Aug;189(2):397–408. doi: 10.1016/0003-9861(78)90227-8. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Lee J. D. Crotoxin, the neurotoxin of South American rattlesnake venom, is a presynaptic toxin acting like beta-bungarotoxin. Naunyn Schmiedebergs Arch Pharmacol. 1977 Jan;296(2):159–168. doi: 10.1007/BF00508469. [DOI] [PubMed] [Google Scholar]
- Damerau B., Lege L., Oldigs H. D., Vogt W. Histamine release, formation of prostaglandin-like activity (SRS-C) and mast cell degranulation by the direct lytic factor (DLF) and phospholipase A of cobra venom. Naunyn Schmiedebergs Arch Pharmacol. 1975;287(2):141–156. doi: 10.1007/BF00510446. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Excell B. J., Patel R., Smith B. Changes in motor end-plates resulting from muscle fibre necrosis and regeneration. A light and electron microscopic study of the effects of the depolarizing fraction (cardiotoxin) of Dendroaspis jamesoni venom. J Neurol Sci. 1974 Apr;21(4):391–417. doi: 10.1016/0022-510x(74)90041-0. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Vane J. R. Prostaglandins: their disappearance from and release into the circulation. Nature. 1967 Dec 2;216(5118):868–873. doi: 10.1038/216868a0. [DOI] [PubMed] [Google Scholar]
- Fohlman J., Eaker D., Karlsoon E., Thesleff S. Taipoxin, an extremely potent presynaptic neurotoxin from the venom of the australian snake taipan (Oxyuranus s. scutellatus). Isolation, characterization, quaternary structure and pharmacological properties. Eur J Biochem. 1976 Sep 15;68(2):457–469. doi: 10.1111/j.1432-1033.1976.tb10833.x. [DOI] [PubMed] [Google Scholar]
- Glavinović M. I. Presynaptic action of curare. J Physiol. 1979 May;290(2):499–506. doi: 10.1113/jphysiol.1979.sp012786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. L., Straight R. Mojave rattlesnake Crotalus scutulatus scutulatus venom: variation in toxicity with geographical origin. Toxicon. 1978;16(1):81–84. doi: 10.1016/0041-0101(78)90065-x. [DOI] [PubMed] [Google Scholar]
- Glenn J. L., Straight R. The midget faded rattlesnake (Crotalus viridis concolor) venom: lethal toxicity and individual variability. Toxicon. 1977;15(2):129–133. doi: 10.1016/0041-0101(77)90031-9. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnakone P., Hawgood B. J. Morphological changes in murine nerve, neuromuscular junction and skeletal muscle induced by the crotoxin complex [proceedings]. J Physiol. 1979 Jun;291:5P–6P. [PubMed] [Google Scholar]
- Habermann E., Breithaupt H. Mini-review. The crotoxin complex--an example of biochemical and pharmacological protein complementation. Toxicon. 1978;16(1):19–30. doi: 10.1016/0041-0101(78)90056-9. [DOI] [PubMed] [Google Scholar]
- Halpert J., Eaker D. Amino acid sequence of a presynaptic neurotoxin from the venom of Notechis scutatus scutatus (Australian tiger snake). J Biol Chem. 1975 Sep 10;250(17):6990–6997. [PubMed] [Google Scholar]
- Harris J. B., Johnson M. A., Macdonell C. Taipoxin, a presynaptically active neurotoxin, destroys mammalian skeletal muscle [proceedings]. Br J Pharmacol. 1977 Sep;61(1):133P–133P. [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Karlsson E., Thesleff S. Effects of an isolated toxin from Australian tiger snake (Notechis scutatus scutatus) venom at the mammalian neuromuscular junction. Br J Pharmacol. 1973 Jan;47(1):141–146. doi: 10.1111/j.1476-5381.1973.tb08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawgood B. J., Smith J. W. The mode of action at the mouse neuromuscular junction of the phospholipase A-crotapotin complex isolated from venom of the South American rattlesnake. Br J Pharmacol. 1977 Dec;61(4):597–606. doi: 10.1111/j.1476-5381.1977.tb07553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenskaya M. A., Thesleff S. The neuromuscular blocking action of an isolated toxin from the elapid (Oxyuranus scutellactus). Acta Physiol Scand. 1974 Apr;90(4):716–724. doi: 10.1111/j.1748-1716.1974.tb05639.x. [DOI] [PubMed] [Google Scholar]
- LENDRUM A. C., FRASER D. S., SLIDDERS W., HENDERSON R. Studies on the character and staining of fibrin. J Clin Pathol. 1962 Sep;15:401–413. doi: 10.1136/jcp.15.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu A. T., Prescott B., Chou C. H., Thomas G. J., Jr Structural properties of mojave toxin of crotalus scutulatus (Mojave rattlesnake) determined by laser Raman spectroscopy. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1139–1145. doi: 10.1016/0006-291x(76)90315-6. [DOI] [PubMed] [Google Scholar]





